Homopiperony lamine 3-methyl-5-chlorine salicylide and synthesis method and application thereof
A technology of piperonyl ethylamine and chlorosalicylaldehyde, which is applied in the field of medicine, can solve problems such as new Schiff bases that have not yet been seen, and achieve good medicinal value, mild reaction conditions, and simple synthesis methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Dissolve 0.01mol of 3-methyl-5-chlorosalicylaldehyde and 0.01mol of piperonylethylamine in 30mL of absolute ethanol (use Molecular sieve dehydration), the obtained solution was refluxed and reacted at 78°C under stirring conditions until complete (TLC tracking detection, about 4h), after the reaction was stopped, the reaction solution was fully cooled to 20°C, and stood for 6h, yellow needle-shaped crystals were precipitated, filtered , the obtained crystals were vacuum-dried at 30° C. for 1 h to obtain a yellow solid product with a yield of 90%.
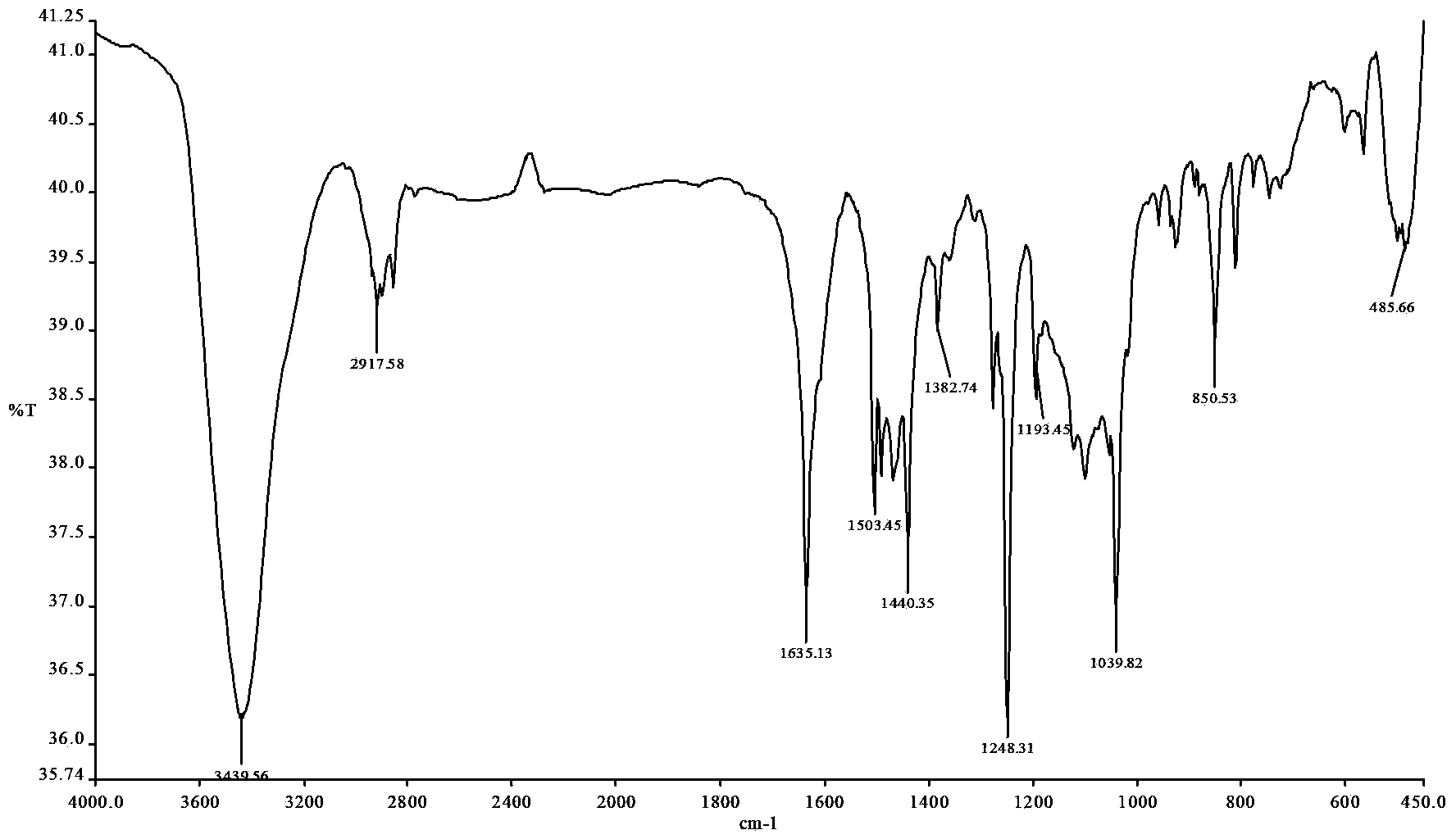
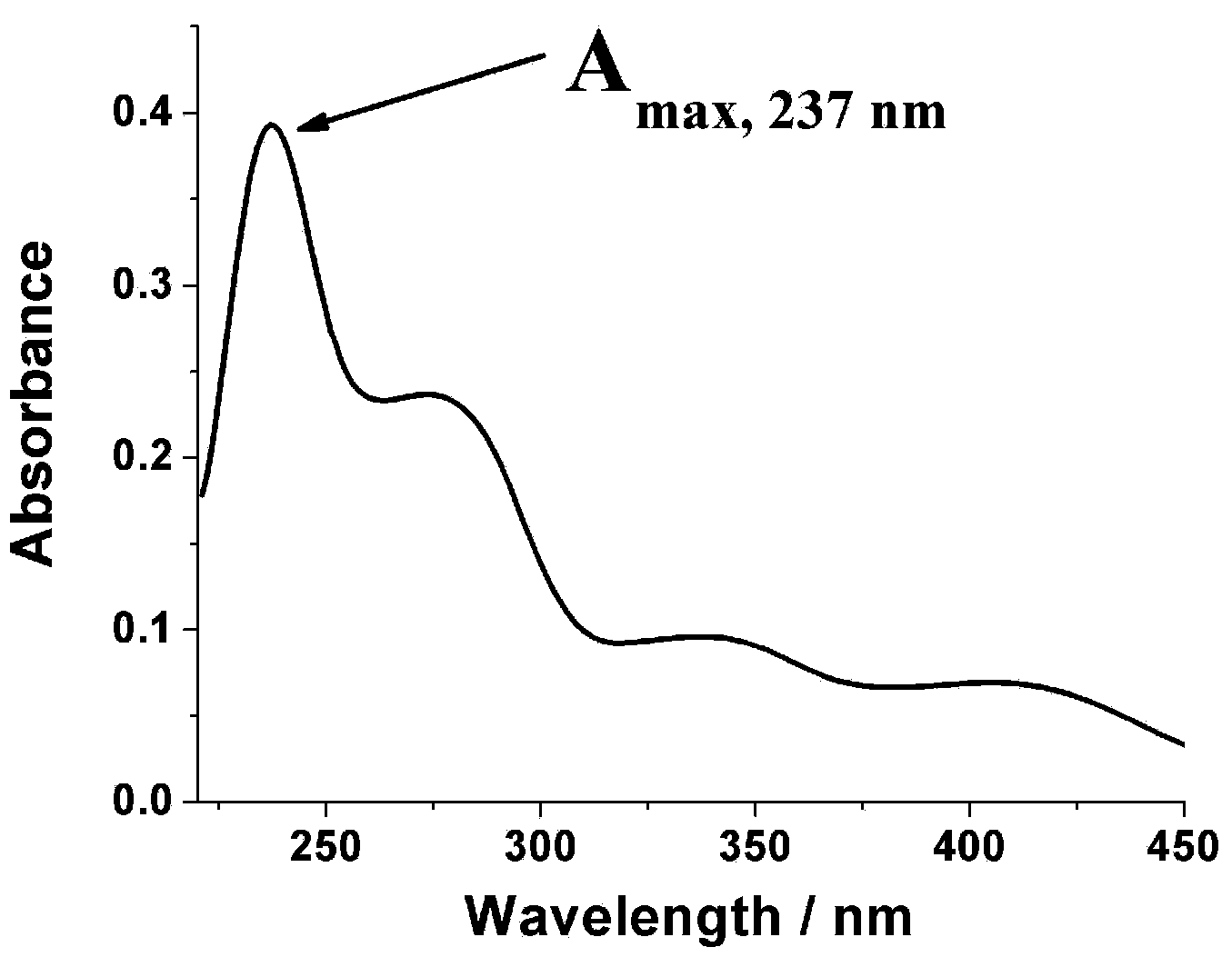
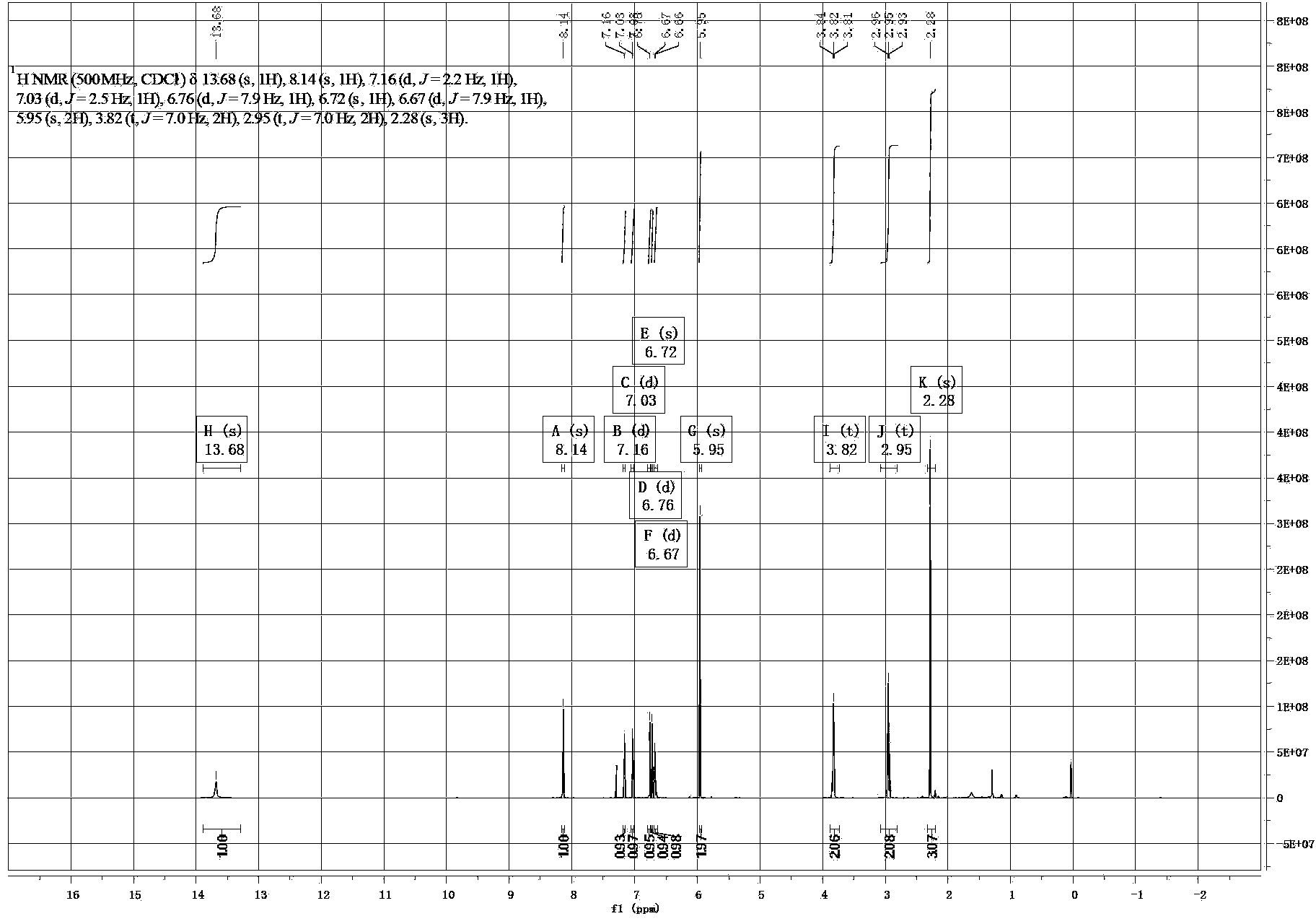
[0025] The yellow solid product obtained above was subjected to infrared spectrum, ultraviolet spectrum, 1 H and 13 C nuclear magnetic resonance spectrum and electrospray mass spectrometry identification, their spectra are as follows figure 1 , 2 , 3, 4 and 5, the specific spectral characteristics are as follows:
[0026] Infrared spectrum: (KBr,cm -1 )3440(ν N-H ),2918(ν Ar-H ),1635(ν C=N ), 1503, 1440 (ν C=C ), 12...
Embodiment 2
[0034] Get 0.01mol of 3-methyl-5-chloro salicylaldehyde and 0.01mol of piperonyl ethylamine and dissolve in 120mL of dichloromethane (dichloromethane is used Molecular sieve dehydration), the resulting solution was stirred and reacted at 45°C until complete (TLC tracking detection, about 6h), after the reaction was stopped, most of the methylene chloride (accounting for 80% of the amount of methylene chloride) was removed by distillation under reduced pressure, and the remaining The reaction solution was fully cooled to 10°C and left to stand for 2 hours. Yellow needle-like crystals were precipitated and filtered. The obtained crystals were vacuum-dried at room temperature for 12 hours to obtain a yellow solid product with a yield of 70%.
[0035] The above-mentioned yellow solid product is subjected to spectroscopic analysis, and its spectral characteristics are the same as the product obtained in Example 1. Therefore, it can be determined that the yellow solid product obtain...
Embodiment 3
[0037] Take 0.01mol of 3-methyl-5-chlorosalicylaldehyde and 0.01mol of piperonyl ethylamine and dissolve in 300mL of chloroform / anhydrous methanol mixed solvent (the volume ratio of chloroform and anhydrous methanol is 2:1, chloroform and Anhydrous methanol before use Molecular sieve dehydration), the obtained solution was refluxed and stirred at 65°C until the reaction was complete (TLC tracking detection, about 1h). The solution was fully cooled to 15°C, left standing for 12h, and yellow needle-like crystals were precipitated, filtered, and the obtained crystals were vacuum-dried at 35°C for 6h to obtain piperonyl ethylamine 3-methyl-5-chlorosalicylaldehyde with a yield of 72%. .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com