Alkaloid compound and preparation method and application of alkaloid compound
A technology of alkaloids and compounds, applied in biochemical equipment and methods, microorganism-based methods, microorganisms, etc., can solve problems such as drugs that have not yet been seen, and achieve the effect of inhibiting cell activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
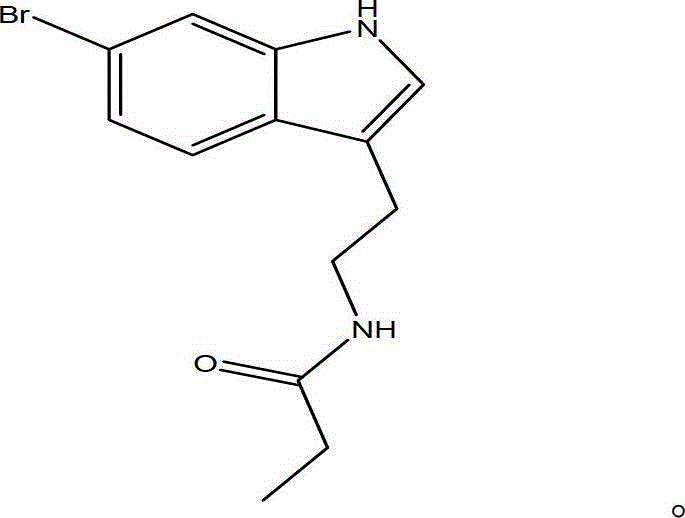
[0022] The structural formula of an alkaloid compound is shown in I.
[0023]
Embodiment 2
[0025] The preparation method of the alkaloid compound shown in formula I specifically includes the following steps:
[0026] (1) Fermentation production
[0027] Pseudoalteromonas rubra.QD1-2 (Pseudoalteromonas rubra.QD1-2) was inoculated on Zobell 2216E solid slant medium by streaking it with the preservation number of CGMCC No.6555, and then cultured in an incubator at 30°C for 1 day; inoculating one colony obtained from slant culture The colonies were placed in 400ml EM medium and cultured on a shaker at 30°C and a rotating speed of 150rpm for 7 days to obtain a fermentation product;
[0028] (2) Obtaining extract
[0029] Centrifuge the fermentation product with a centrifuge to remove the bacteria to obtain the fermentation broth. Take the fermentation broth and add an equal volume of ethyl acetate to fully extract once, collect the extract, and then add the raffinate to an equal volume of ethyl acetate and repeat the extraction once. , Collect the extract, add the obtained raff...
Embodiment 3
[0041] In vitro anti-tumor activity test (cell proliferation inhibitory activity test)
[0042] (1) Experimental samples
[0043] Preparation of the test sample solution The test sample is the pure compound I separated and purified in the above-mentioned Example 1. An appropriate amount of the sample is accurately weighed, and a solution of the required concentration is prepared with methanol to test the activity.
[0044] The cell line and cell subculture adopt human gastric adenocarcinoma BGC-823 cells, human cervical cancer Hela cells and human liver cancer HepG2 cells and other cancer cell lines. All cells use 10% BCS 1640 medium at 37℃. Enter 0.5% carbon dioxide incubator for relay culture.
[0045] (2) Experimental method
[0046] Cell proliferation inhibitory activity test method: the tetrazolium salt (MTT) method takes human gastric adenocarcinoma BGC-823 cells, human cervical cancer Hela cells and human liver cancer HepG2 cells in logarithmic growth phase, and adjusts the dens...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com