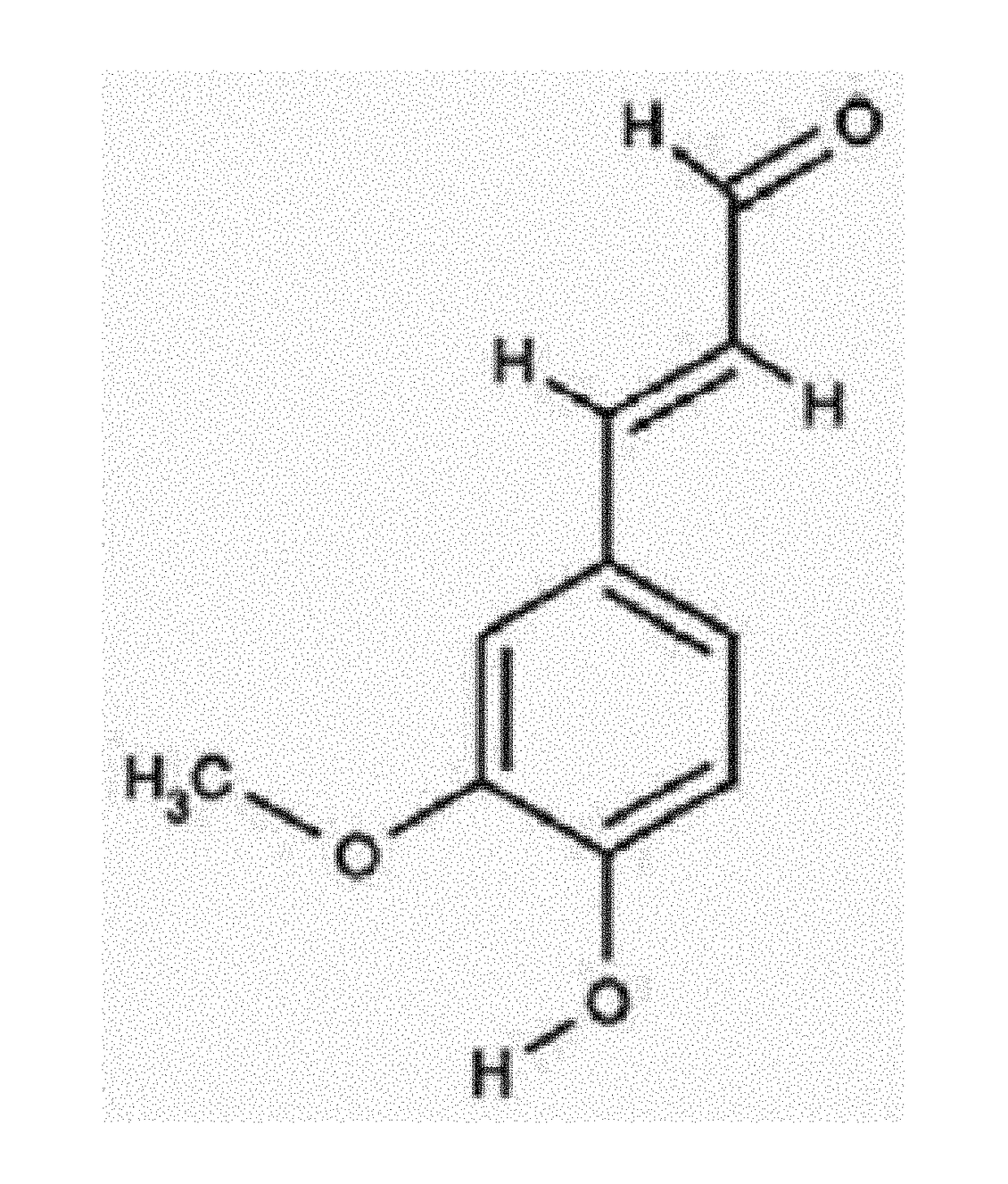
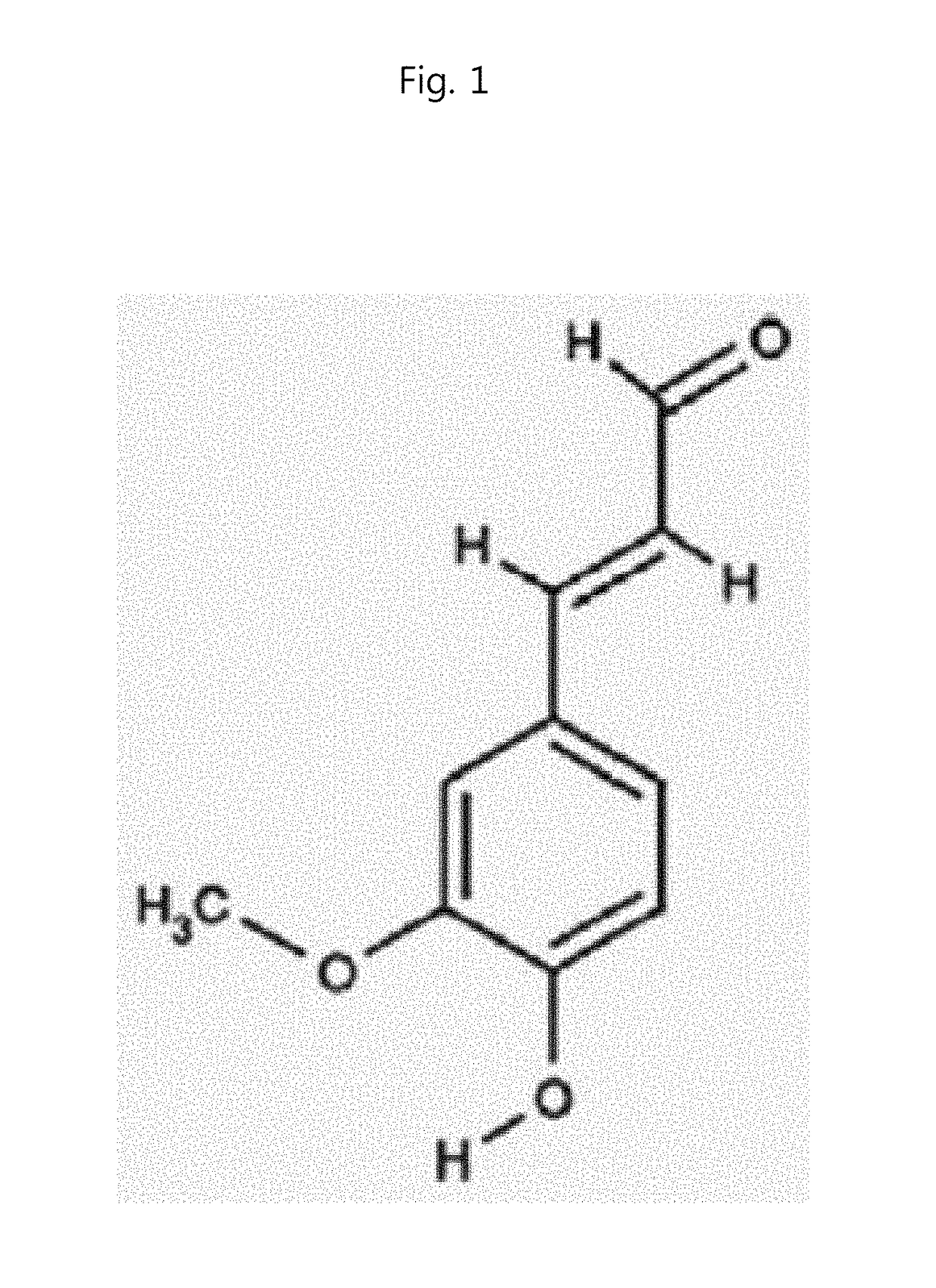
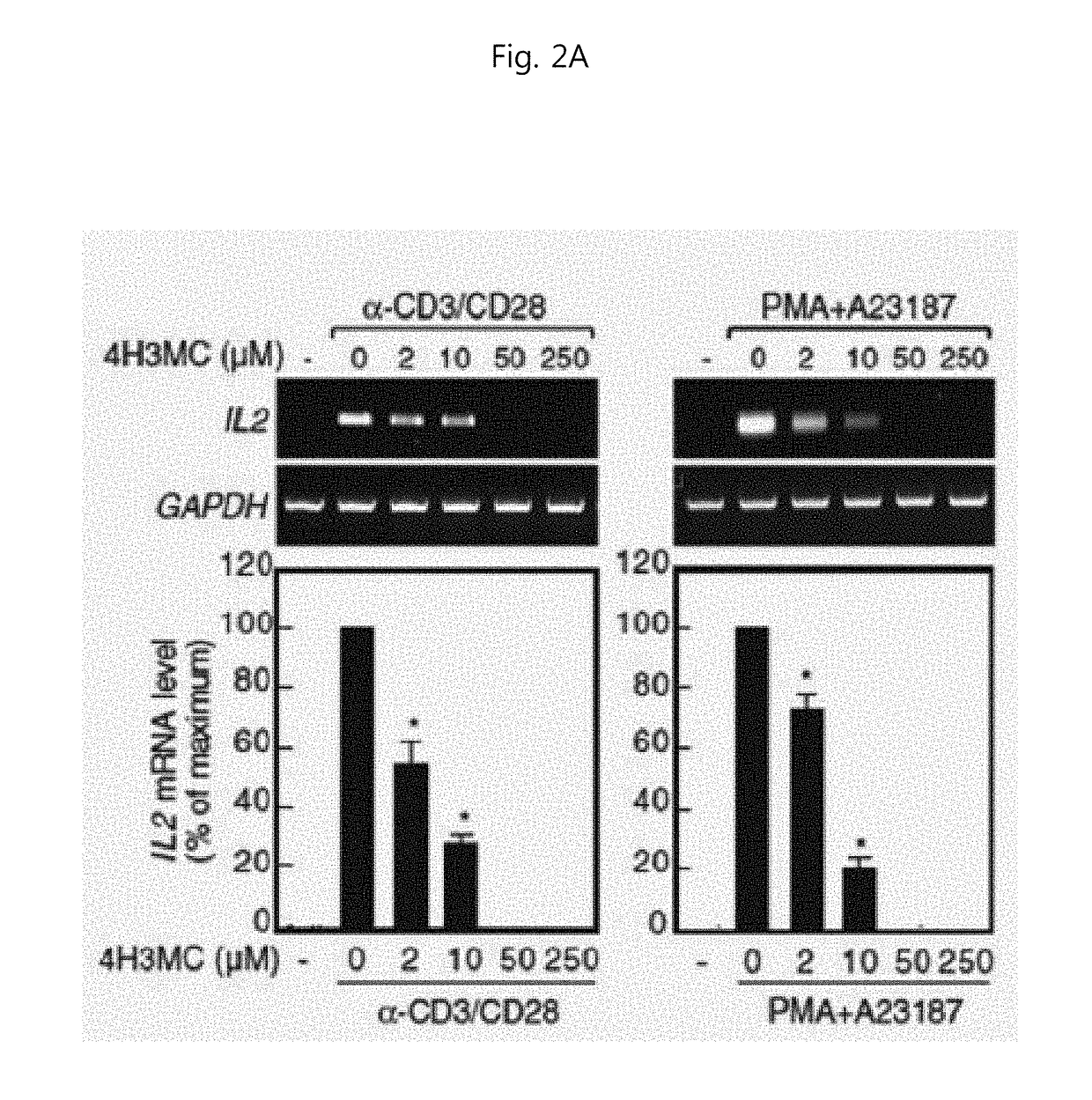
Pharmaceutical Compositions for Preventing and Treating Th1 or Th2 mediated Immune Disease Comprising 4H3MC(4-Hydroxy-3-methoxycinnamaldehyde) as an Active Ingredient
a technology of th1 or th2mediated immune disease and active ingredient, which is applied in the direction of pharmaceutical delivery mechanism, medical preparations, edible oils/fats, etc., can solve the problems of undeveloped therapeutic agent against t-cell-mediated disease, which targets pkc, and the effect of 4h3mc, a cinnamic acid derivative, on the activation of t cells,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0051]Materials and Methods
[0052]Cell Culture
[0053]Jurkat T cells (ATCC, CRL-1651, Manassas, Va.) and Raji B cells (ATCC, CCL-86) were cultured in RPMI complete medium supplemented with 10% fetal bovine serum (FBS, AusGeneX, Santa Clara, Calif.) and PenStrep (Gibco-RBL). After written informed consent, human peripheral blood leukocytes (PBL) were isolated from healthy donors by a sedimentation method using dextran and Ficoll Amersham Biosciences, Piscataway, N.J.). All the cell lines and cells used in the present invention were cultured at 37° C. in a humidified incubator containing 5% CO2. All experiments using human peripheral blood leukocytes were approved by the Ethics Committee of the School of Life Sciences, GIST.
[0054]Reagents and Antibodies
[0055]4-Hydroxy-3-methoxycinnamaldehyde (4H3MC; PubChem CID: 5280536) and p-hydroxycinnamic acid (HCA; PubChem CID: 637542) with the purity over 99% were provided by Professor Seung-Ho Lee from Yeungnam University (Korea). For treatment in...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentrations | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com