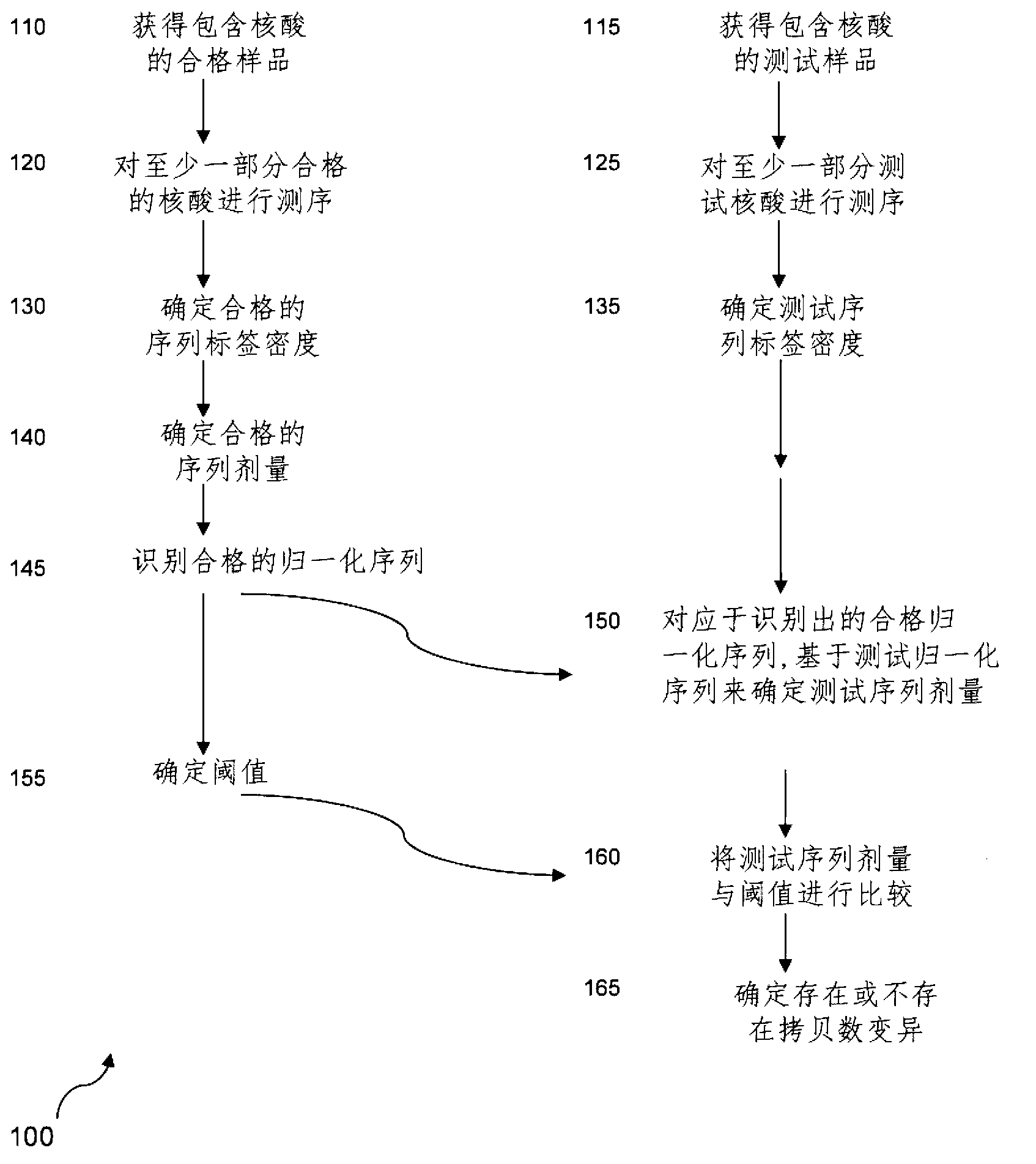
Method for determining the presence or absence of different aneuploidies in a sample
An aneuploidy, sample technique, used in diagnostics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0264] Sample Processing and DNA Extraction
[0265] Peripheral blood samples were collected from multiple pregnant women considered at risk for fetal aneuploidy during their first and second trimesters. Consent was obtained from each participant prior to blood draw. Blood was collected prior to amniocentesis or chorionic villus sampling. Karyotyping was performed using chorionic villus or amniocentesis samples to confirm fetal karyotyping.
[0266] Peripheral blood drawn from each subject was collected in ACD tubes. Transfer one tube of blood sample (approximately 6-9 mL / tube) to a 15-mL low-speed centrifuge tube. Blood was centrifuged at 2640 rpm, 4°C for 10 minutes using a Beckman Allegra 6R centrifuge and rotor model GA 3.8.
[0267]For cell-free plasma extraction, the supernatant plasma was transferred to a 15-mL high-speed centrifuge tube and centrifuged at 16000 x g, 4°C for 10 minutes using a Beckman Coulter Avanti J-E centrifuge with a JA-14 rotor. These two cent...
example 2
[0274] Dosage and variation for chromosomes 13, 18, 21, X, and Y
[0275] To examine the extent of interchromosomal variability and intersequence determination variability in the number of mapped sequence tags for all chromosomes, plasma cfDNA obtained from the peripheral blood of 48 volunteer pregnant subjects was extracted and as in example The instructions in 1 were sequenced and analyzed as follows.
[0276] The total number of sequence tags mapped to each chromosome (sequence tag density) was determined. Alternatively, the number of mapped sequence tags can be normalized to the length of the chromosome to yield a sequence tag density ratio. Normalization to chromosome length is not a required step, but can be done separately to reduce the number of digits in a number, thereby simplifying it for human interpretation. Chromosome lengths that can be used to normalize these sequence tag counts can be those provided at the world wide web site genome.ucsc.edu / goldenPath / stats...
example 3
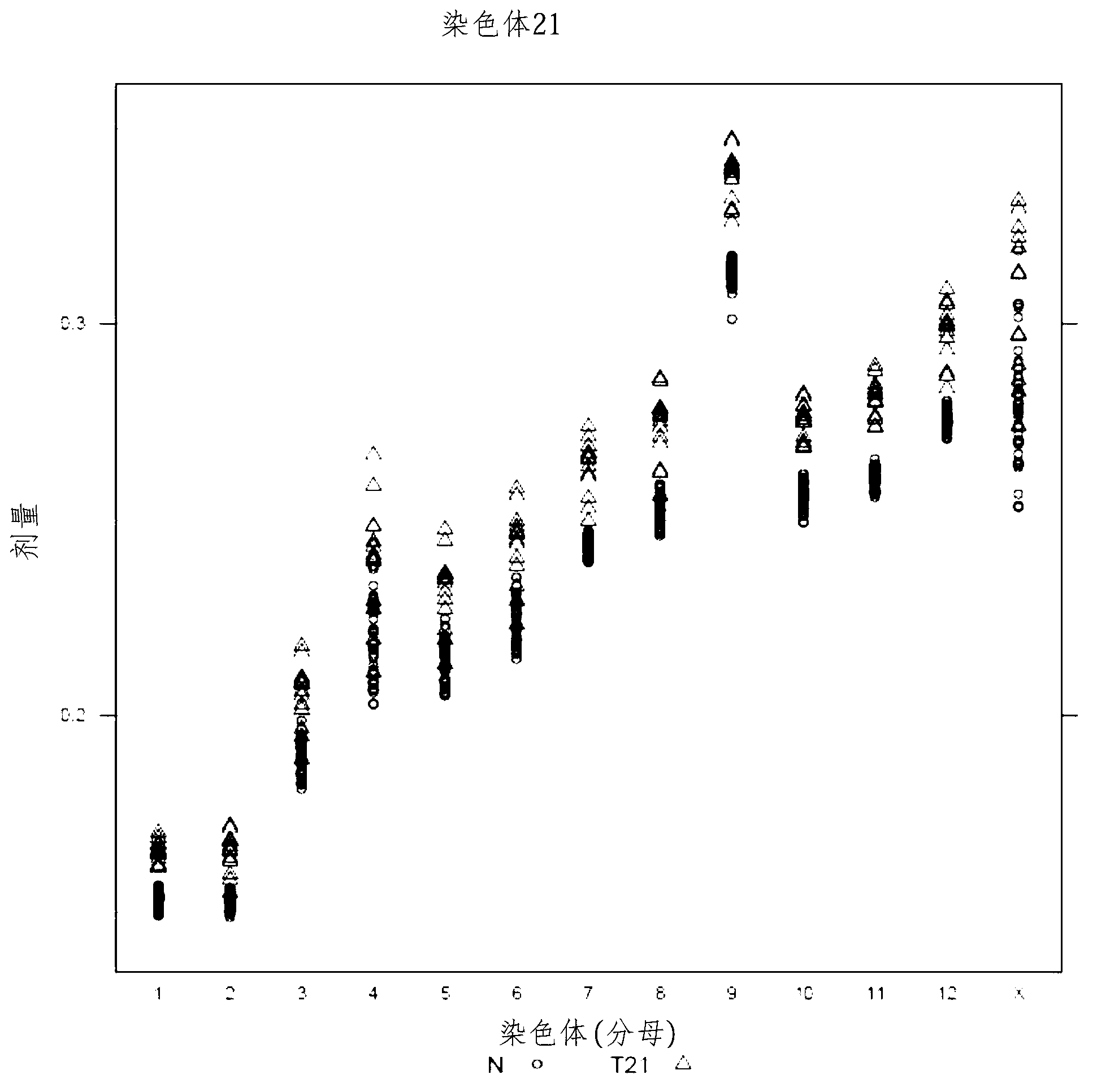
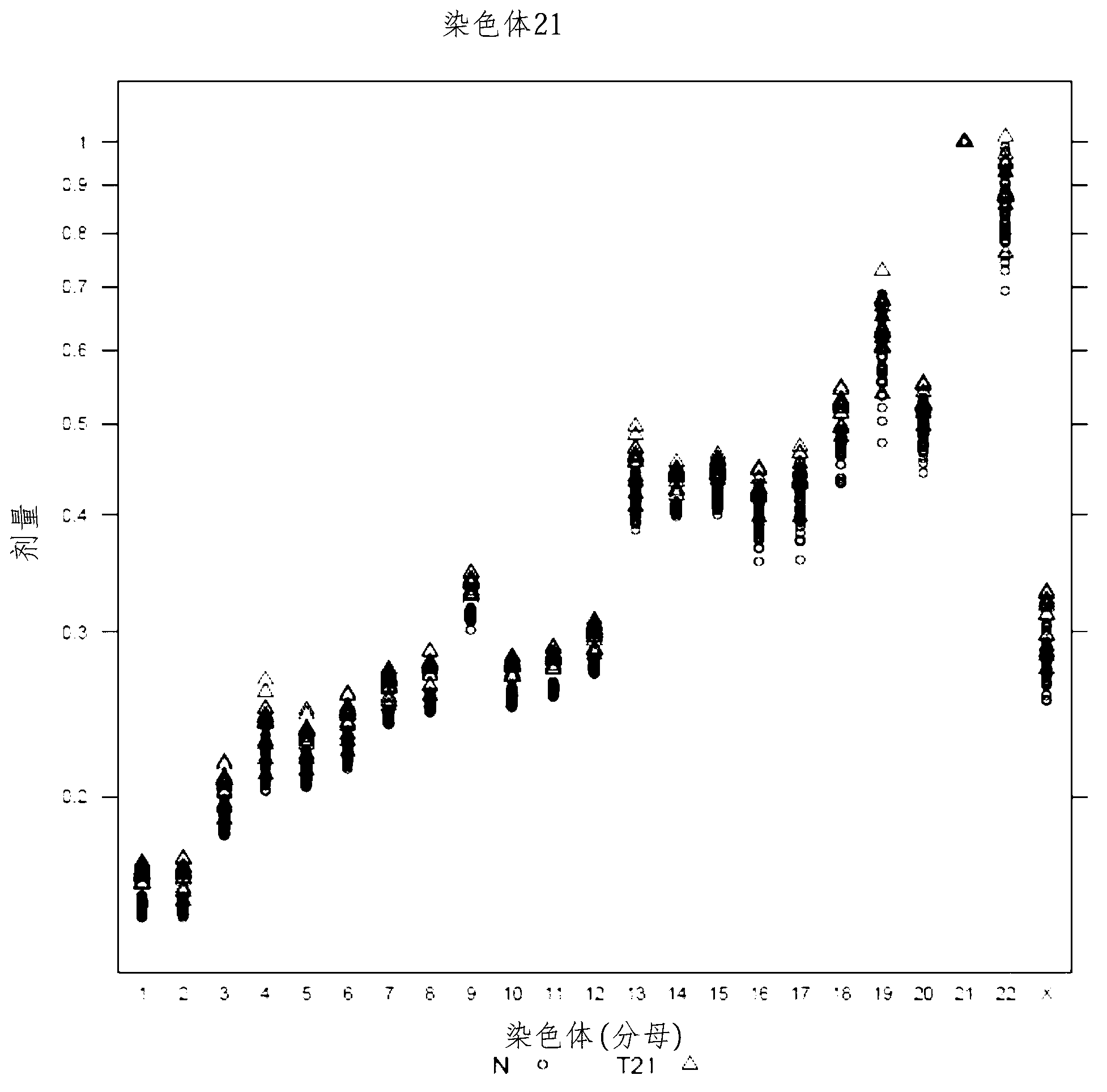
[0295] Fetal aneuploidy diagnosed using normalizing chromosomes
[0296] To implement the use of chromosome dosage to assess aneuploidy in biological test samples, maternal blood test samples were obtained from pregnant volunteers and cfDNA was prepared, sequenced and analyzed as described in Examples 1 and 2.
[0297] Trisomy 21
[0298] Table 4 provides the calculated doses for chromosome 21 in an exemplary test sample (#11403). The calculated threshold for a positive diagnosis of T21 was set at >2 standard deviations from the mean of these qualified (normal) samples. The diagnosis of T21 is given based on the chromosome dose in the test sample being greater than a set threshold. Chromosomes 14 and 15 were used as normalizing chromosomes in separate calculations to show that either the chromosome with the lowest variability (e.g., chromosome 14) or the chromosome with the greatest discriminability (e.g., chromosome 15) could be used to identify aneuploidy. Thirteen T21 s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com