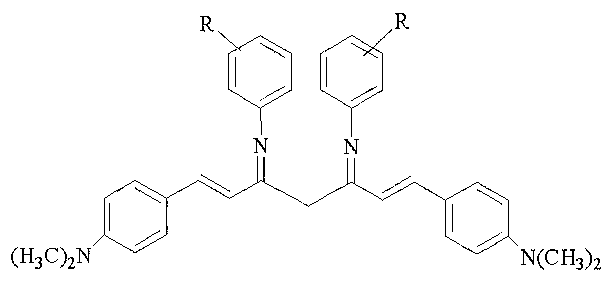
Curcuminoid condensed aromatic amine Schiff base derivative, as well as preparation method and application thereof in preparation of antibacterial medicaments
A technology of aromatic amine Schiff base and curcuminoids, which is applied in the field of synthesizing curcuminoid condensed 3, 5-diarylamine Schiff base derivatives, can solve the problems of low bioavailability and poor water solubility of curcumin, and achieve Good antibacterial effect, long antibacterial effective time, improved bioavailability and antibacterial activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Example 1: Curcuminoid derivatives 1,7-bis(4-dimethylaminophenyl)-3,5-bis(4-fluorophenylimino)-1,6-heptadiene (DAEF) synthesis.
[0017] Add 2 mmol of 4-fluoroaniline in a three-necked flask, dissolve in 5 mL of absolute ethanol, then add catalytic amount of formic acid and anhydrous aluminum chloride, and slowly add 1 mmol of the above product curcumin analog DAEO and 12 mL of anhydrous aluminum chloride dropwise under stirring. Mixed solution of water and ethanol, heated to reflux reaction, nitrogen protection, reacted for 15 hours, and thin-layer chromatography detected the reaction. After the reaction, the organic phase was extracted, concentrated, and separated by silica gel thin-layer chromatography (petroleum ether: ethyl acetate = 2:1), a yellow solid product was obtained with a yield of 12.4%. And through FTIR, MALDI-TOP, 1 HNMR and other methods confirmed the structure of the compound. IR(KBr) ν: 3046, 2982, 2841, 1645, 1633, 1500-1600, 1355, 1233cm -1 Ma...
Embodiment 2
[0018] Embodiment 2: curcuminoid derivative 1,7-bis(4-dimethylaminophenyl)-3,5-bis(4-chlorophenylimino)-1,6-heptadiene (DAECl) synthesis.
[0019] Add 2mmol of 4-chloroaniline in a three-necked flask, dissolve in 5mL of absolute ethanol, then add catalytic amount of formic acid and anhydrous aluminum trichloride, and slowly add 1mmol of the above-mentioned product curcumin analog DAEO and 15mL of anhydrous aluminum chloride dropwise under stirring. Mixed solution of water and ethanol, heated to reflux reaction, nitrogen protection, reacted for 8 hours, and thin-layer chromatography detected the reaction. After the reaction, the organic phase was extracted, concentrated, and separated by silica gel thin-layer chromatography (petroleum ether: ethyl acetate = 3:1), a yellow solid product was obtained with a yield of 22.7%. And through FTIR, MALDI-TOP, 1 HNMR and other methods confirmed the structure of the compound. IR(KBr) ν: 3036, 2979, 2839, 1642, 1631, 1490-1580, 1350, 733...
Embodiment 3
[0020] Example 3: Curcuminoid derivative 1,7-bis(4-dimethylaminophenyl)-3,5-bis(4-bromophenylimino)-1,6-heptadiene (DAEBr) synthesis.
[0021] Add 2 mmol of 4-bromoaniline in a three-necked flask, dissolve in 5 mL of absolute ethanol, then add catalytic amount of formic acid and anhydrous aluminum chloride, and slowly add 1 mmol of the above product curcumin analog DAEO and 15 mL of anhydrous aluminum chloride dropwise under stirring. The mixed solution of water and ethanol was heated to reflux for reaction, protected by nitrogen, and reacted for 3 hours. Thin-layer chromatography detected the reaction. After the reaction, the organic phase was extracted and separated by silica gel thin-layer chromatography (petroleum ether: ethyl acetate=4: 1), to obtain a tan solid product with a yield of 34.7%. And through FTIR, MALDI-TOP, 1 HNMR and other methods confirmed the structure of the compound. IR(KBr) ν: 3032, 2966, 2834, 1640, 1627, 1488-1575, 1346, 623cm -1 Mass Spectrum M...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com