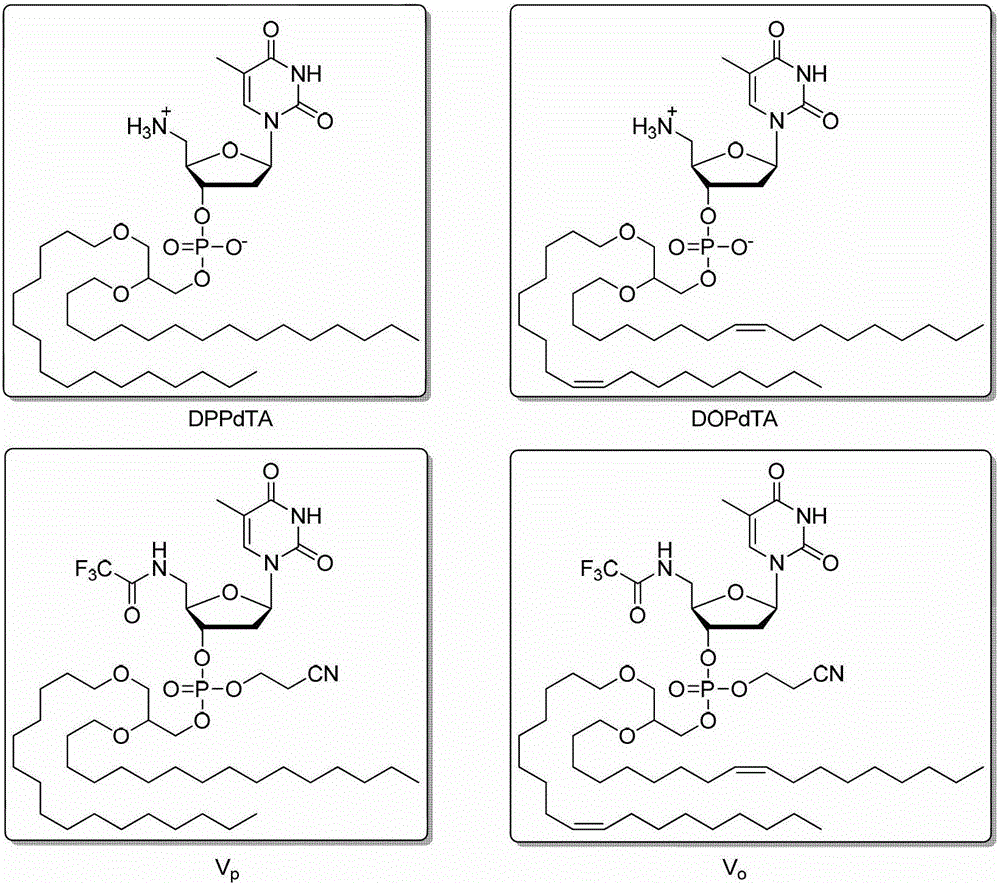
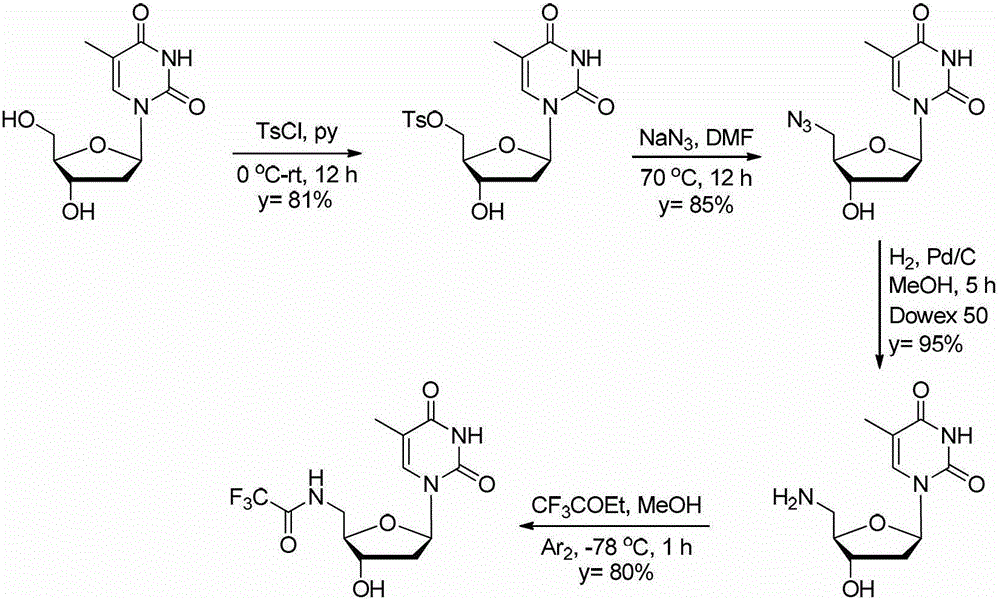
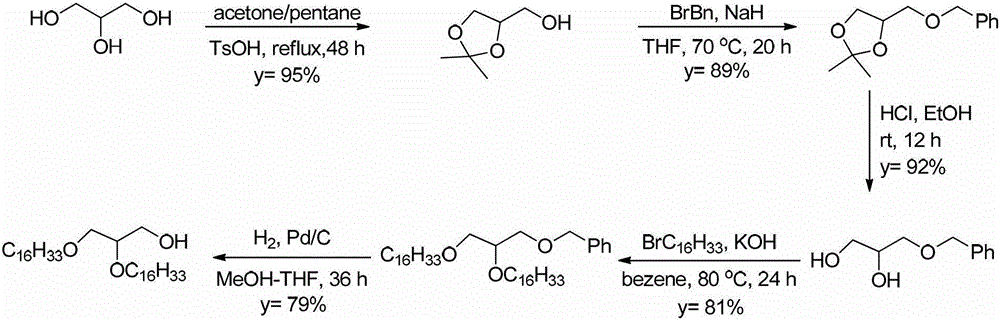
5'-amino-2', 5'-dideoxynucleotide phospholipid molecule and its preparation method and application
A technology of deoxynucleotide phospholipids and deoxynucleosides, applied in other methods of inserting foreign genetic materials, preparation of sugar derivatives, chemical instruments and methods, etc., can solve the problems of difficult effective release, high cytotoxicity of cationic liposomes and serum Toxicity and other issues, to achieve the effect of good efficacy, simple and efficient synthesis method, and simple preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] [Example 1] Synthesis of 5'-toluenesulfonyl ester thymidine
[0067] Thymidine (24.2 g, 0.1 mol) was dissolved in anhydrous pyridine (200 mL), and cooled to 0° C. in an ice bath. p-Toluenesulfonyl chloride (23 g, 0.12 mol) was dissolved in anhydrous pyridine (100 mL), and slowly added dropwise to the aforementioned reaction solution through a microsampler, and the dropping process lasted for 6 hours. The reaction process was always carried out at 0°C under the protection of argon. After the dropwise addition was completed, the reaction was returned to room temperature, and stirring was continued for 6 h. Pyridine was distilled off under reduced pressure. To the residue was added ethyl acetate 500 mL and NaHCO 3 Aqueous solution (10%) 300mL, a large amount of white solid precipitated. After filtration, the obtained white solid is the target product. The filtrate was collected, the organic phase was separated, and the aqueous phase was washed with 300 mL of ethyl ace...
Embodiment 2
[0068] [Example 2] Synthesis of 5'-azido-2',5'-dideoxythymidine
[0069] Dissolve 5'-p-methylsulfonylcarbothymidine (5g, 12.6mmol) in anhydrous DMF (25mL), add NaN 3 (1.2g, 19mmol), heated to 70°C, and reacted for 12h under the protection of argon. The solvent was evaporated under reduced pressure. Dichloromethane (50 mL) was added to the residue, followed by washing with water (30 mL). Anhydrous Na for organic phase 2 SO 4 Dry and separate by silica gel column chromatography, eluent DCM:MeOH=20:1, to obtain 2.9 g of the target product (yield 85%). white solid. 1 HNMR (400MHz, DMSO-d 6):δ=11.31(s,1H),7.49(s,1H),6.20(t,J=7.2Hz,1H),5.39(d,J=4.0Hz,1H),4.20(s,1H),3.79 -3.85(m,1H),3.56(d,J=5.2Hz,2H),2.20-2.30(m,1H),2.05-2.15(m,1H),1.79(s,3H); 13 CNMR (100MHz, DMSO-d 6 ):δ=163.7, 150.5, 136.1, 109.8, 84.6, 83.9, 70.7, 51.6, 38.1, 12.1; 1298.8, 1272.8, 1067.3, 963.4, 856.2, 636.4, 553.4, 493.8cm -1 ;MS(ESI-TOF+)forC 10 h 13 N 5 o 4 Na[M+Na] + found290.1042,calcd290.08...
Embodiment 3
[0070] [Example 3] Synthesis of 5'-amino-2',5'-dideoxythymidine
[0071] Take 5'-azido-2',5'-dideoxythymidine (5.5g, 20mmol) and dissolve it in methanol (150mL), add 10% palladium carbon catalyst (0.55g), and place it in a hydrogenation apparatus hydrogenation. The pressure is 60Psi. After hydrogenation at room temperature for 5 h, the reaction was stopped. TLC detection found that the raw material had reacted completely. Suction filtration through celite under reduced pressure to remove the solid matter, and the obtained filtrate was evaporated to dryness to obtain 4.8 g of light yellow solid. The solid is insoluble in organic solvents such as methanol and ethyl acetate, and is very polar, and cannot be separated by ordinary silica gel column chromatography. The resulting solid was dissolved in water and purified by strongly acidic ion exchange resin Dowex50 to obtain 4.7 g of the target product with a yield of 95%. white solid. 1 HNMR (400MHz, DMSO-d 6 ):δ=7.65(s,1H),...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com