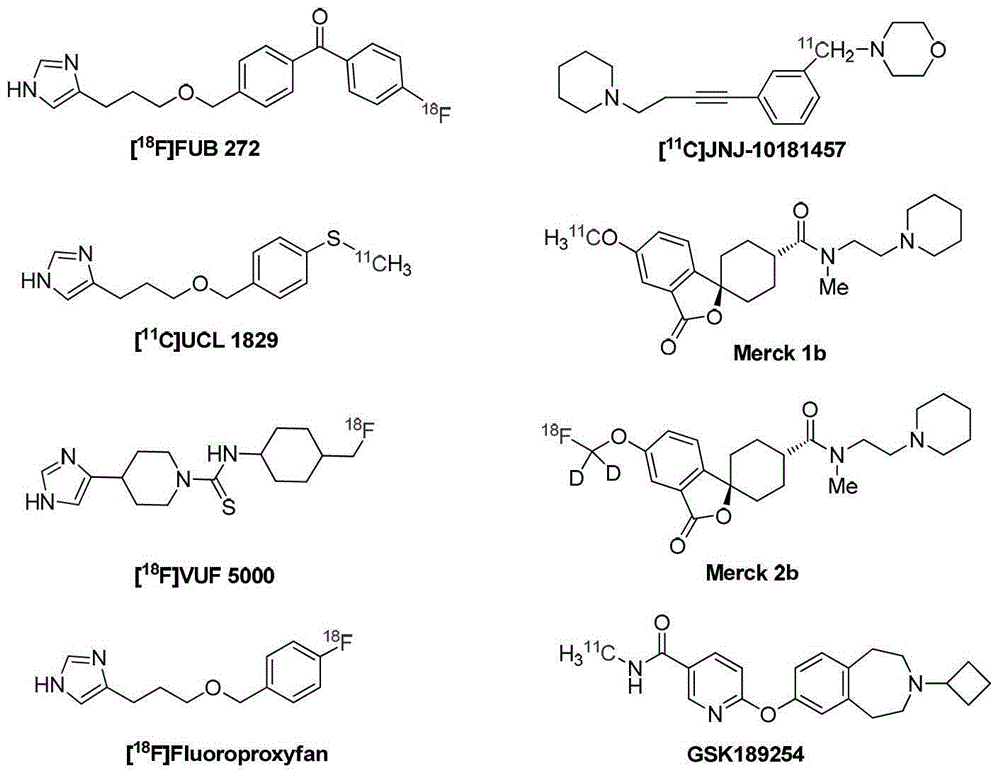
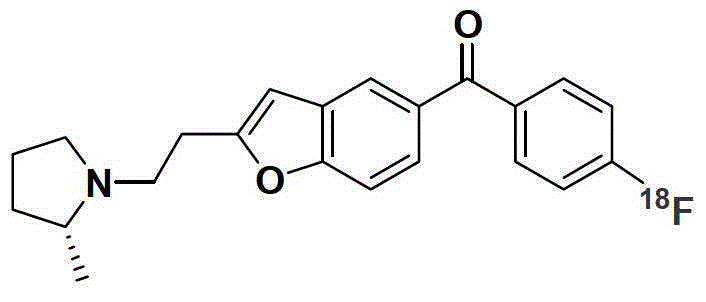
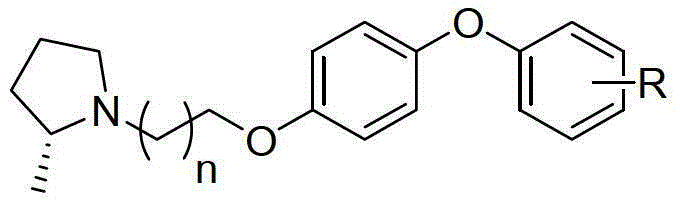
A precursor of brain histamine H3 receptor radioligand and its preparation method
A technology of radioactivity and brain histamine, applied in the direction of organic chemistry, can solve the problems of multiple reaction steps, high non-specific binding, and low radiochemical yield, and achieve the goal of less harsh reaction conditions, improved binding force, and easy separation and purification Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] A kind of brain histamine H of the present invention 3 The preparation method of the precursor of receptor radioligand, concrete steps are as follows:
[0033] Step 1, adding fluoro or nitrophenoxyphenol and 1-bromo-3-chloropropane or 1-bromo-2-chloroethane into the acetonitrile organic solvent, stirring to dissolve, then adding anhydrous potassium carbonate, Heating to 85-90°C, reflux for 24-48 hours, cooling, extraction, drying, concentration, and flash column chromatography to obtain the product of the first step; wherein, fluoro or nitrophenoxyphenol and 1-bromo-3- The molar ratio of chloropropane or 1-bromo-2-chloroethane is 1:1~2; the molar ratio of fluoro or nitrophenoxyphenol to potassium carbonate is 1:2~5; fluoro or nitrobenzene The molar ratio of oxyphenol to acetonitrile is 1:0.22~0.44; the nitro or fluorine of the fluorinated or nitrophenoxyphenol is the 2nd or 4th position, and the preferred scheme is that R is the 2nd nitro Or the 4th nitro group or the...
Embodiment 1
[0038] 1. At room temperature, add 4-(2-nitrophenoxy)phenol (500mg, 2.16mmol), potassium carbonate (597mg, 4.32mmol) and 25ml of acetonitrile into a 50mL round bottom flask, and slowly add 1-Bromo-3-chloropropane (0.427 mL, 4.32 mmol). The reaction mixture was heated to 85° C., condensed and refluxed and stirred for 38 h (TLC thin layer chromatography followed the reaction progress). Cooling, acetonitrile was removed by distillation under reduced pressure, potassium carbonate was extracted, the solution was concentrated, and flash column chromatography gave product 1-(4-(3-chloropropoxy)phenoxy)-2-nitrobenzene 573mg, the yield It was 86.1%, and the product was a yellow oily substance.
[0039] The product is identified by hydrogen spectrum and carbon spectrum, and the detection results are as follows:
[0040] 1 HNMR (500MHz, CDCl 3 ):δ7.94(1H,dd,J 1 =1.50Hz,J 1 =8.15Hz),7.46(1H,m),7.14(1H,m),7.03(2H,m),6.93(3H,d,J=8.85Hz),4.12(2H,t,J=5.80Hz), 3.77(2H,t,J=6.30Hz),2.26(2...
Embodiment 2
[0048] 1. At room temperature, add 4-(2-nitrophenoxy)phenol (1g, 4.32mmol), potassium carbonate (4.48g, 21.6mmol) and 30ml of acetonitrile in a 50mL round bottom flask, then slowly drop Add 1-bromo-2-chloroethane (0.358 mL, 4.32 mmol). The reaction mixture was heated to 85° C., condensed and stirred under reflux for 33 h (TLC thin layer chromatography followed the reaction progress). Cooling, acetonitrile was removed by distillation under reduced pressure, potassium carbonate was extracted, the solution was concentrated, and flash column chromatography gave product 1-(4-(2-chloroethoxy)phenoxy)-2-nitrobenzene 555mg, the yield was 43.8%, and the product was a pale yellow oily substance.
[0049] The product is identified by hydrogen spectrum and carbon spectrum, and the detection results are as follows:
[0050] 1 HNMR (CDCl 3 ):δ7.92(1H,dd,J 1 =1.60Hz,J 2 =8.15Hz),7.45(1H,m),7.13(1H,m),7.02(2H,m),6.92(3H,m),4.22(2H,t,J=5.80Hz),3.82(2H,t ,J=5.85Hz)ppm.
[0051] 13 CNMR...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com