Application of pingyangmycin combined with sodium hyaluronate in drug for treating lymphatic malformation
A sodium hyaluronate and lymphatic malformation technology, applied in drug combinations, medical preparations with non-active ingredients, antineoplastic drugs, etc., can solve problems such as poor effect, unsatisfactory clinical effect, and increased pulmonary fibrosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The preparation of a kind of medicine that is used for the treatment of lymphatic malformation involved in the present invention:
[0029] Take pingyangmycin 8mg, sodium hyaluronate 20mg, add the pharmaceutically acceptable carrier that prepares injection, prepare 6mL injection. Since the fluid in the lymphangioma cannot be completely pumped out and replaced with drugs of this concentration, the actual concentrations of pingyangmycin and sodium hyaluronate injected into the tumor are about 100 μg / mL and 300 μg / mL.
[0030] Sodium hyaluronate is commercially available sodium hyaluronate injection, Shandong Bausch & Lomb Freda Pharmaceutical Co., Ltd., approval number: National Drug Accreditation H10960136.
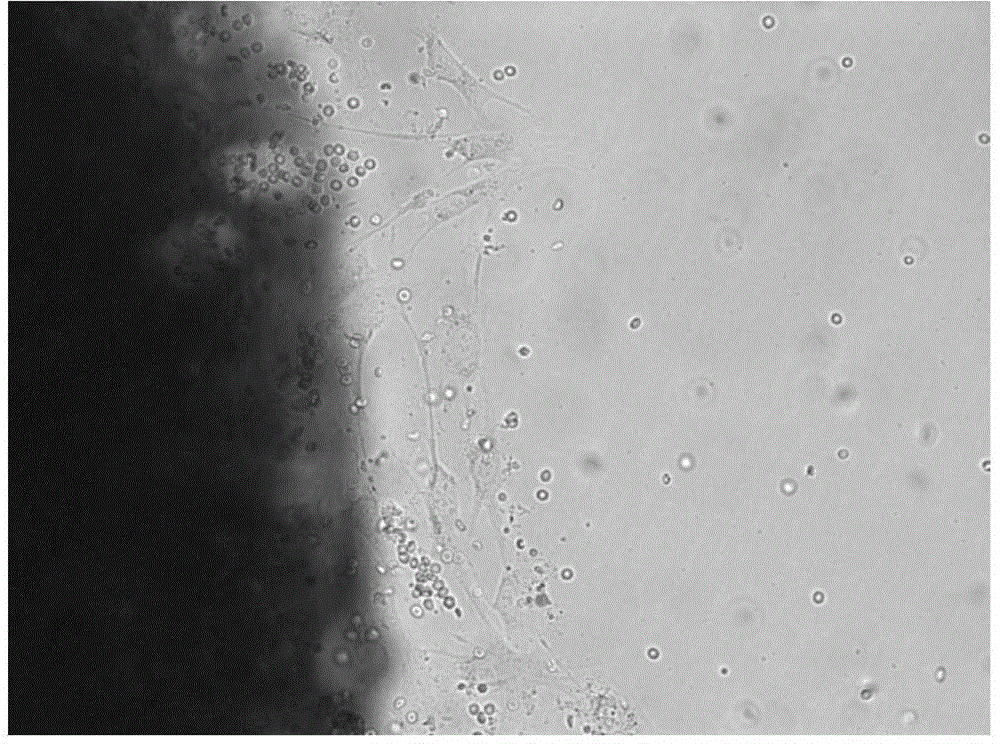
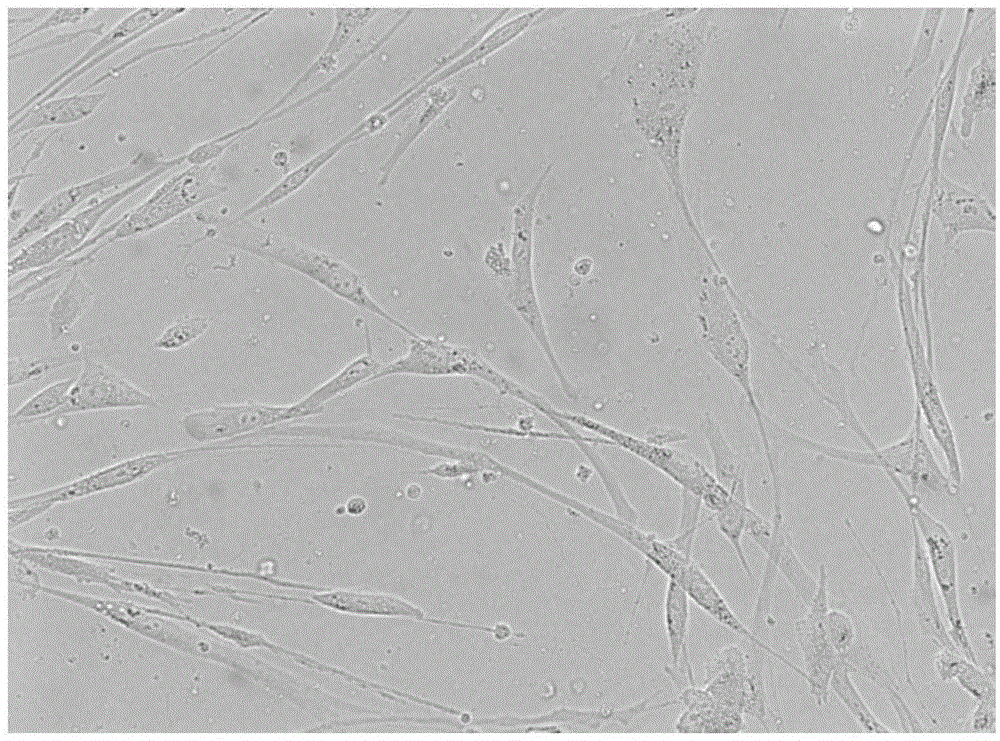
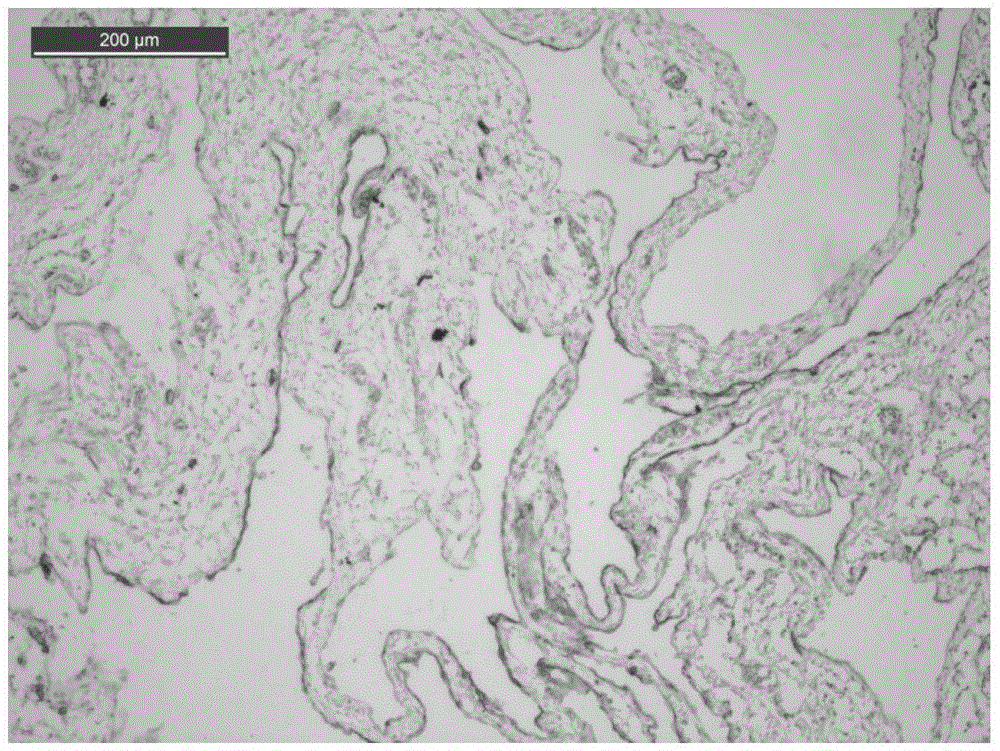
[0031] The following pharmacodynamic experiments will further illustrate its drug activity.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com