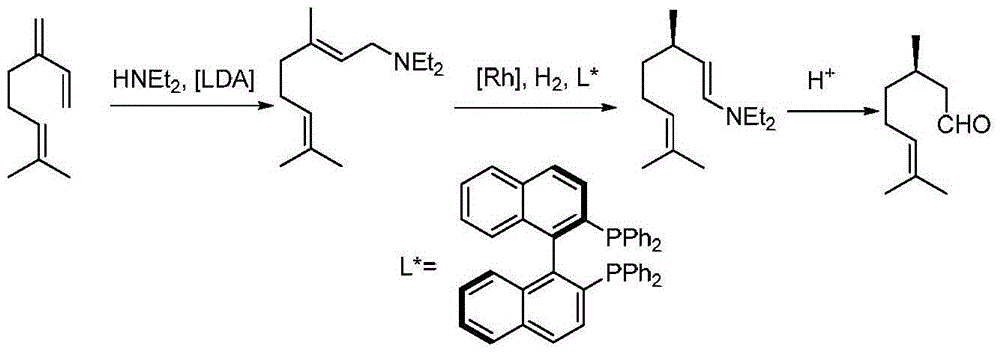
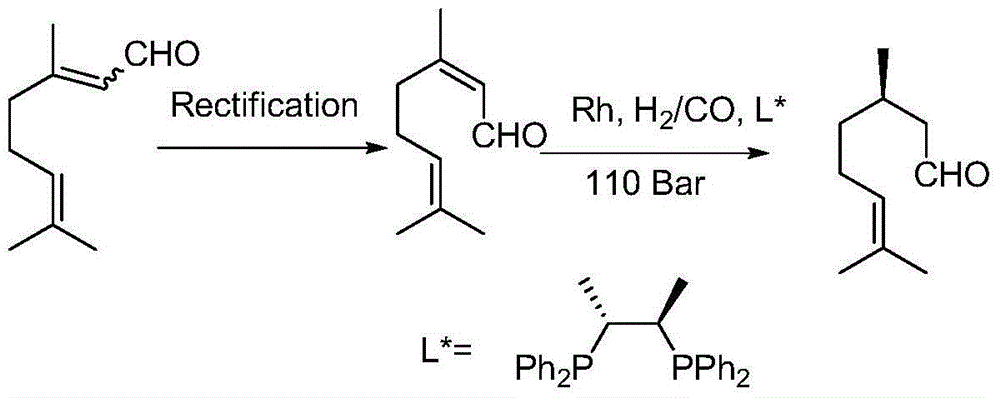
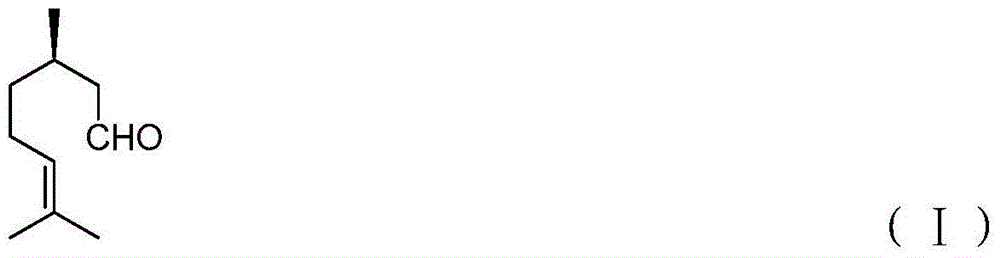Immobilized asymmetric catalyst and application thereof in asymmetric hydrogenation reaction
A hydrogenation reaction, asymmetric technology, applied in physical/chemical process catalysts, organic compound/hydride/coordination complex catalysts, preparation of organic compounds, etc., can solve the problem of catalyst recycling, limited application value, production The problem of high cost has achieved the effect of broad industrial application value, easy recycling and application, and low production cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~18
[0061] According to the raw material ratio of Table 1, take by weighing 2 grams of citral, Pd / BaSO 4 . II-7 Immobilized chiral adjuvant, trifluoroacetic acid (42mg), solvent (10mL) were placed in a 50ml three-necked flask, and stirred at a certain temperature for 1 hour under nitrogen protection. Then, the reaction was continued for 20 hours by replacing the nitrogen with a hydrogen balloon, and then the catalyst was removed by filtration, and the obtained filtrate was analyzed by gas chromatography.
[0062] The conversion rate and the selectivity of reaction gained citronellal are shown in Table 1:
[0063] Table 1
[0064]
Embodiment 19~47
[0066] Weigh 2 grams of citral, Pd / BaSO 4 (56mg), immobilized chiral adjuvant L (160mg), trifluoroacetic acid (42mg), tert-amyl alcohol (10mL) in a 50ml three-necked flask, under nitrogen protection, stirred at a certain temperature for 1 hour. Then use hydrogen balloon to replace nitrogen and continue to react for 20 hours. The catalyst was then removed by filtration and the filtrate obtained was analyzed by gas chromatography.
[0067] Table 2
[0068]
[0069]
Embodiment 48~58
[0071] The catalyst recovery method is: add 5 mL of ethyl acetate to dilute and stir at room temperature for 0.5 to 1.0 hours, filter out the pyrrolidine immobilized catalyst, wash the filter cake with ethyl acetate and dichloromethane successively, dry under reduced pressure at room temperature and continue Proceed to the next batch of catalytic reactions. The effect of applying 10 batches of reactions is shown in Table 3:
[0072] table 3
[0073]
[0074]
[0075] The reaction conditions of Examples 48 to 58 in Table 3 are: take 2 grams of citral, and the Pd / BaSO4 obtained from the last batch of treatment 4 and II-7 catalyst, 42 mg of trifluoroacetic acid, and tert-amyl alcohol (10 mL) were placed in a 50 ml three-necked flask, and stirred at 60° C. for 1 hour under nitrogen protection. Then use hydrogen balloon to replace nitrogen and continue to react for 20 hours. Then it was cooled to room temperature, and 1 mL of triethylamine was added dropwise, and then the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com