Patents
Literature
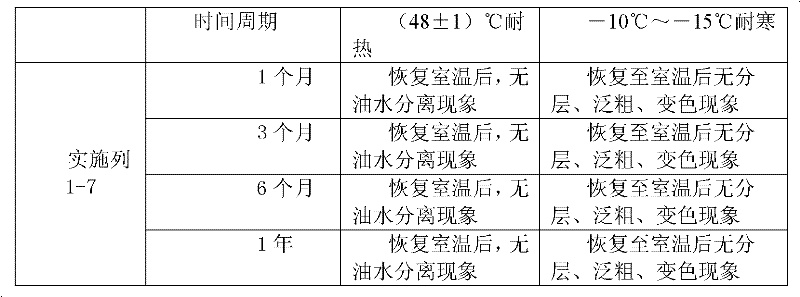
159 results about "Imidazolidines" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Compounds based on reduced IMIDAZOLINES which contain no double bonds in the ring.
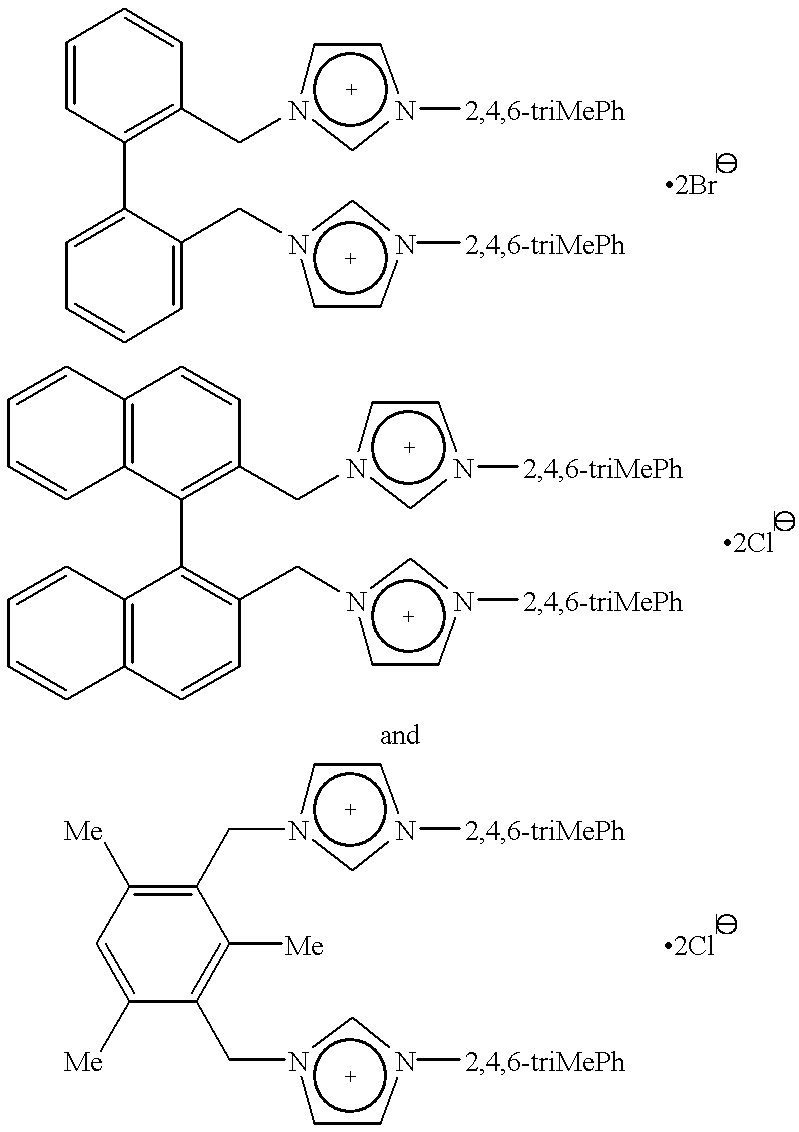
Catalyst system comprising transition metal and imidazoline-2-ylidene or imidazolidine-2-ylidene
InactiveUS6316380B1Inexpensive and readily synthesizedHigh yieldCarboxylic acid esters preparationOrganic grignard reactionsCarbeneImidazolidines
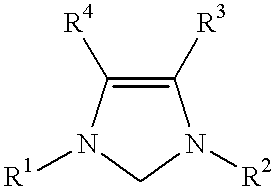
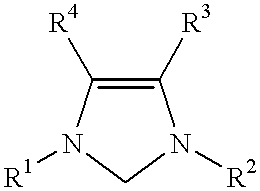
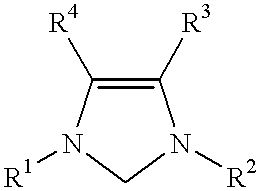
This invention provides a catalyst system useful in many coupling reactions, such as Suzuki, Kumada, Heck, and amination reactions. The catalyst system of the present invention makes use of N-heterocyclic carbenes or their protonated salts. The composition of the catalyst system comprises at least one transition metal compound and at least one N-heterocyclic carbene or its protonated salt. This invention further provides novel N-heterocyclic carbenes and their protonated salts. One type of N-heterocyclic carbene used in this invention is an imidazolinc-2-ylidene wherein the 1 and 3 positions are each, independently, substituted by an aromatic group in which each ortho position is, independently, substituted by a secondary or tertiary group which has at least three atoms.
Owner:NEW ORLEANS RES & TECH FOUND UNIV OF +1
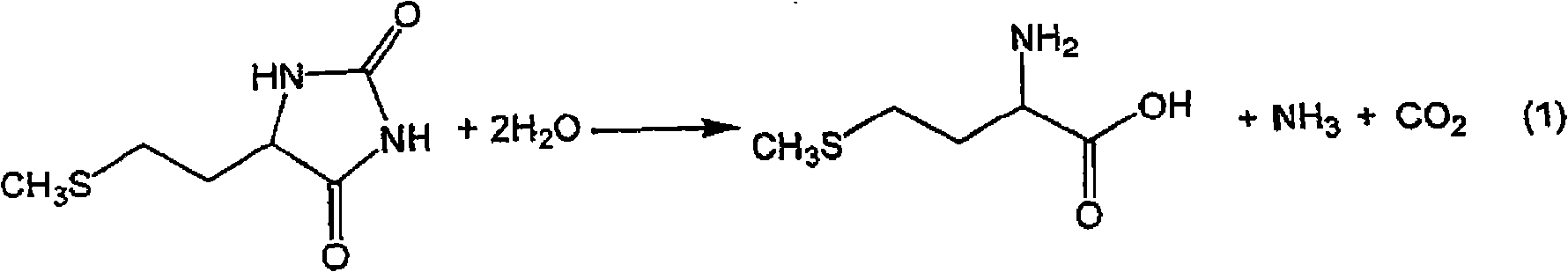
Process for producing methionine
InactiveCN101602700AEfficient recyclingEfficient productionOrganic compound preparationSulfide preparationPotassiumSlurry
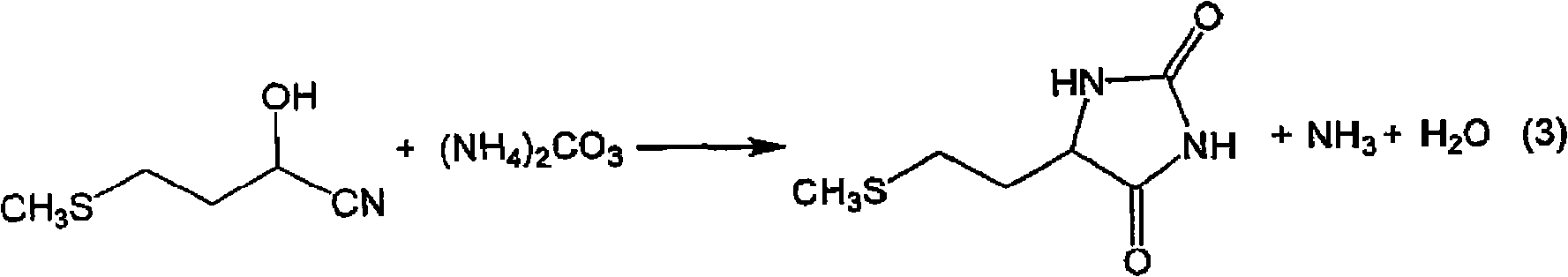
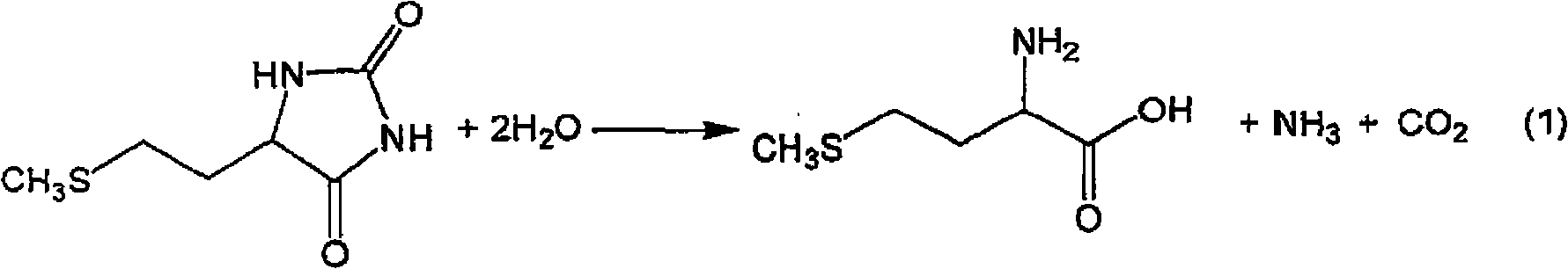
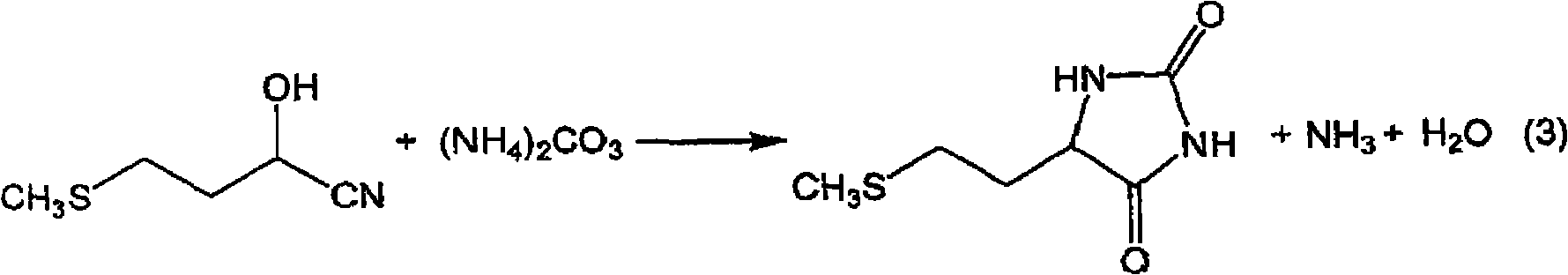
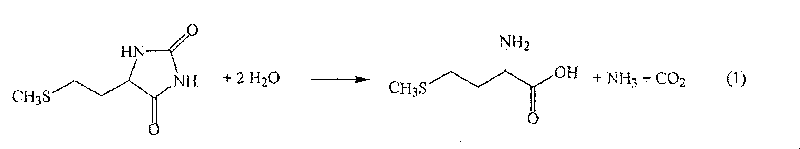
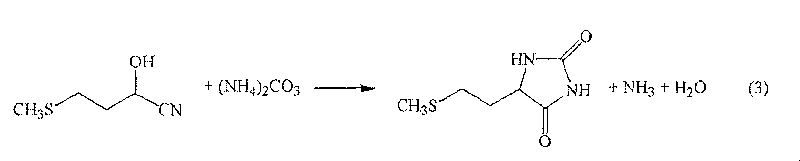
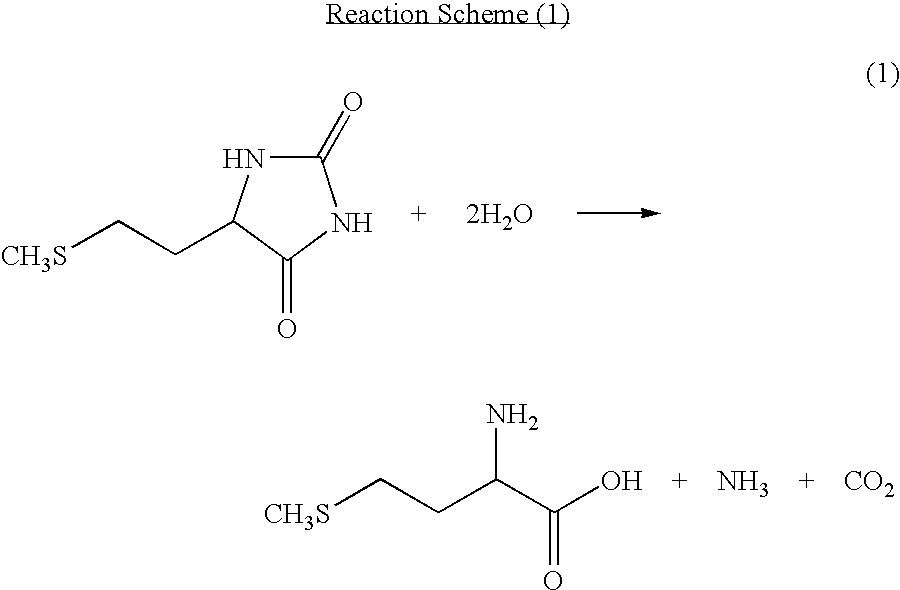
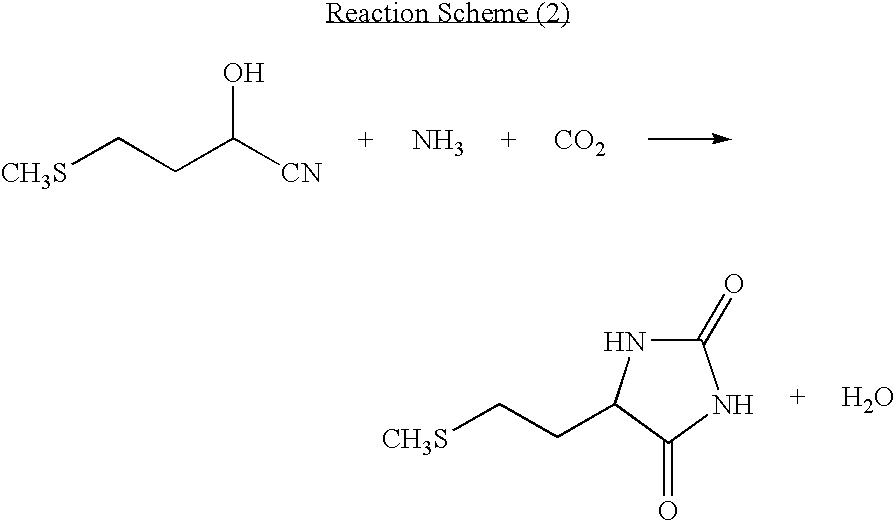
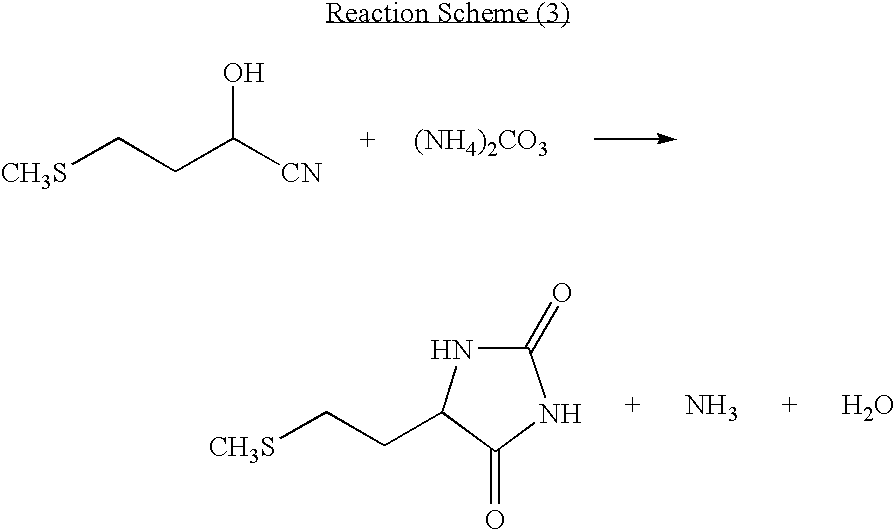
The present invention provides a process for producing methionine which is advantageous from the viewpoints of cost and wastewater disposal, and which comprises the following steps (1) to (5): (1) a reaction step comprising hydrolysis of 5-[2-(methylthio)ethyl]imidazolidine-2,4-dione in the presence of a basic potassium compound; (2) a first crystallization step comprising introduction of carbon dioxide into the reaction solution obtained in step (1) to precipitate methionine, and separation of the resultant slurry into a precipitate and a mother liquor; (3) a second crystallization step comprising concentration of the mother liquor obtained in step (2), mixing with a lower alcohol, introduction of carbon dioxide into the mixture to precipitate methionine and potassium hydrogen carbonate, and separation of the resultant slurry into a precipitate and a mother liquor; (4) a heating step comprising concentration of the mother liquor obtained in step (3) followed by a heating treatment at 150 to 200 DEG C; and (5) a third crystallization step comprising introduction of carbon dioxide into the heated mother liquor obtained in step (4) to precipitate methionine and potassium hydrogen carbonate, and separation of the resultant slurry into a precipitate and a mother liquor.
Owner:SUMITOMO CHEM CO LTD
Process for producing methionine
InactiveCN101602701AReduce the final amountOrganic compound preparationSulfide preparationPotassiumMethionine biosynthesis
The present invention provides a process for producing methionine, which comprises the steps of: hydrolyzing 5-[2-(methylthio)ethyl]imidazolidine-2,4-dione in the presence of a basic potassium compound in a non-stirred continuous first reaction tank, and heat-treating the reaction solution after hydrolysis in a second reaction tank. According to the process of the present invention, a methionine crystal with a higher bulk density can be produced.
Owner:SUMITOMO CHEM CO LTD
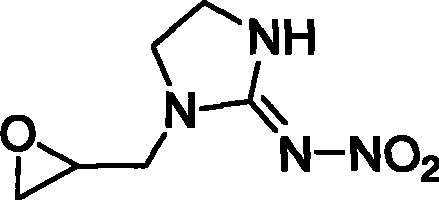
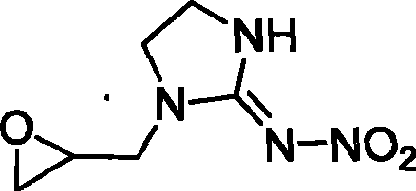
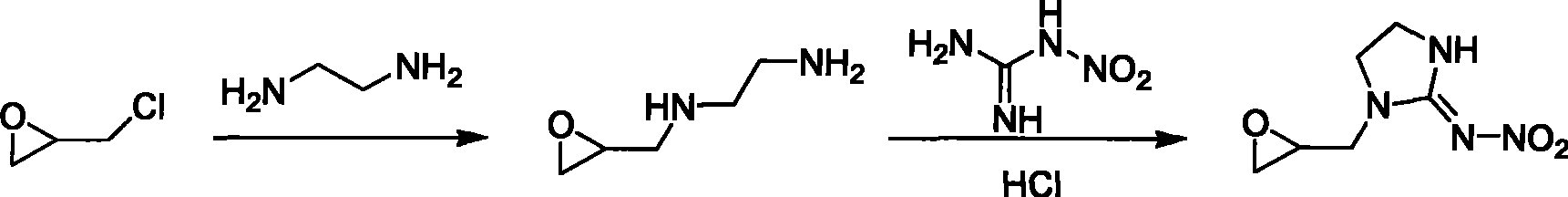
1-(1,2-epoxy propyl)-n-nitroimidazolene amine-2, preparation and use thereof
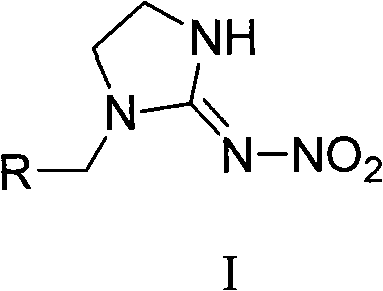
The invention relates to 1-(1,2-epoxypropyl)-N-nitroimidazoline-2-amine which has a structural formula as shown in the picture. A preparation method of the compound comprises the steps of adding ethylenediamine to a reaction container, dropwise adding epoxy chloropropane in the reaction container while stirring, reacting for 2-3h, cooling, and vaporizing out acetonitrile to obtain epoxypropyl ethylenediamine; taking and dissolving nitroguanidine and the epoxypropyl ethylenediamine into water, dropwise adding hydrochloric acid solution while stirring, reacting for 2-3h, cooling, extracting, desolventizing to obtain crude product, and recrystallizating to obtain the product; or adding N-nitroimidazoline, potassium carbonate and organic solvent to the reaction container, heating to 75-81.6 DEG C, dropwise adding epoxy chloropropane in the reaction container after dissolving the N-nitroimidazoline, continuously heating until completing the reaction after dropwise adding, cooling reaction products, filtering, vaporizing filtrate to dry, adding water to residues, extracting by using methylene dichloride, desolventizing to obtain phase product, and recrystallizating by using butanone to obtain the product.
Owner:河北艾林化工科技有限公司
Sterilizing and algae-removing agent and application thereof
InactiveCN102578131AEfficient sterilization and algae killing performanceReduce dosageBiocideDisinfectantsIsothiazolinoneEpoxiconazole
The invention discloses a sterilizing and algae-removing agent and an application thereof. The sterilizing and algae-removing agent consists of the following components in parts by weight: 100 parts of dodecyl dimethyl benzyl ammonium chloride, 4-6 parts of sulfamic acid, 60-80 parts of isothiazolinone, 10-20 parts of crylic acid-acrylic ester-sulfonate terpolymer, 60-80 parts of attapulgite clay, 0.2-0.4 parts of 4,4' dichloro-2-hydroxy diphenyl oxide, 4-6 parts of dodecyl ethoxy imidazolidine, 10-18 parts of sodium dichloro isocyanurate, 10-18 parts of methane dithiocyanate and 1-2 parts of epoxiconazole. The sterilizing and algae-removing agent has the advantages of efficient sterilizing and algae-removing performance, small using amount, safety, nontoxicity and environmental friendliness. The sterilizing and algae-removing agent is applied to sterilizing treatment of a circular water system.
Owner:SHANGHAI TAOHONG CHEM TECH
Use of a catalyst system comprising nickel palladium or platinum and imidazoline-2-ylidene or imidazolidine-2-ylidene in stille coupling reactions
InactiveUS6362357B1High yieldEliminate needHydrocarbon by isomerisationOrganic compound preparationLiquid mediumOxidation state
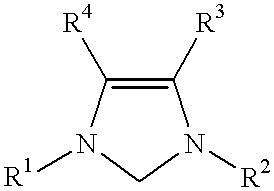
This invention provides a process for conducting Stille coupling reactions. The processes of the present invention make use of N-heterocyclic carbenes as ancillary ligands in Stille couplings of aryl halides. A Stille coupling can be carried out by mixing, in a liquid medium, at least one strong base; at least one aryl halide or aryl pseudohalide in which all substituents are other than stannyl groups, wherein the aryl halide has, directly bonded to the aromatic ring(s), at least one halogen atom selected from the group consisting of a chlorine atom, a bromine atom, and an iodine atom; at least one organotin compound wherein the tin atom is quaternary, wherein one group bound to the tin atom is unsaturated at the alpha or beta position, and wherein each of the remaining groups bound to the tin atom is a saturated group; at least one metal compound comprising at least one metal atom selected from nickel, palladium, and platinum, wherein the formal oxidation state of the metal is zero or two; and at least one N-heterocyclic carbene. One preferred type of N-heterocyclic carbene is an imidazoline-2-ylidene of the formulawherein R1 and R2 are each, independently, alkyl or aryl groups having at least 3 carbon atoms, R3 and R4 are each, independently, a hydrogen atom, a halogen atom, or a hydrocarbyl group.
Owner:RES & TECH FOUND UNIV OF NEW ORLEANS
Cream for chap removal
InactiveCN104546562APrevent chappingCracked isolationCosmetic preparationsToilet preparationsGlycerolOil phase
The invention discloses a cream for chap removal, which is composed of a water phase, an oil phase and a functional phase, wherein in parts by weigh, the water phase is composed of 45-85 parts of water, 0.1-0.5 part of allantoin and 0.05-0.2 part of diazolidinyl urea, the oil phase is composed of 0.5-1.5 parts of cetostearyl alcohol, 1-3 parts of glyceryl stearate, 0.5-1.5 parts of PEG-100 stearate, 2.5-5 parts of glycerin, 1-5 parts of polydimethylsiloxane, 2.5-5 parts of propylene glycol, 1-3 parts of isopropyl palmitate, 1-3 parts of petrolatum, 0.5-1.5 parts of polysorbate-60, 5-15 parts of mineral oil, 0.1-0.3 part of methylparaben, and 0.05-0.15 part of propyl hydroxybenzoate; and the functional phase is composed of 1-4 parts of one or more of ammonium polyacrylate, C13-16 isoparaffin, and laureths-25, 0.15-0.45 part of essence and 0.25-0.75 part of vitamin E. Experiments show that the cream both has the functions of water locking and moisturizing and has the effects of chap prevention and chap removal, therefore, the cream has a good market prospect.
Owner:无锡樱花梦美容制品有限公司
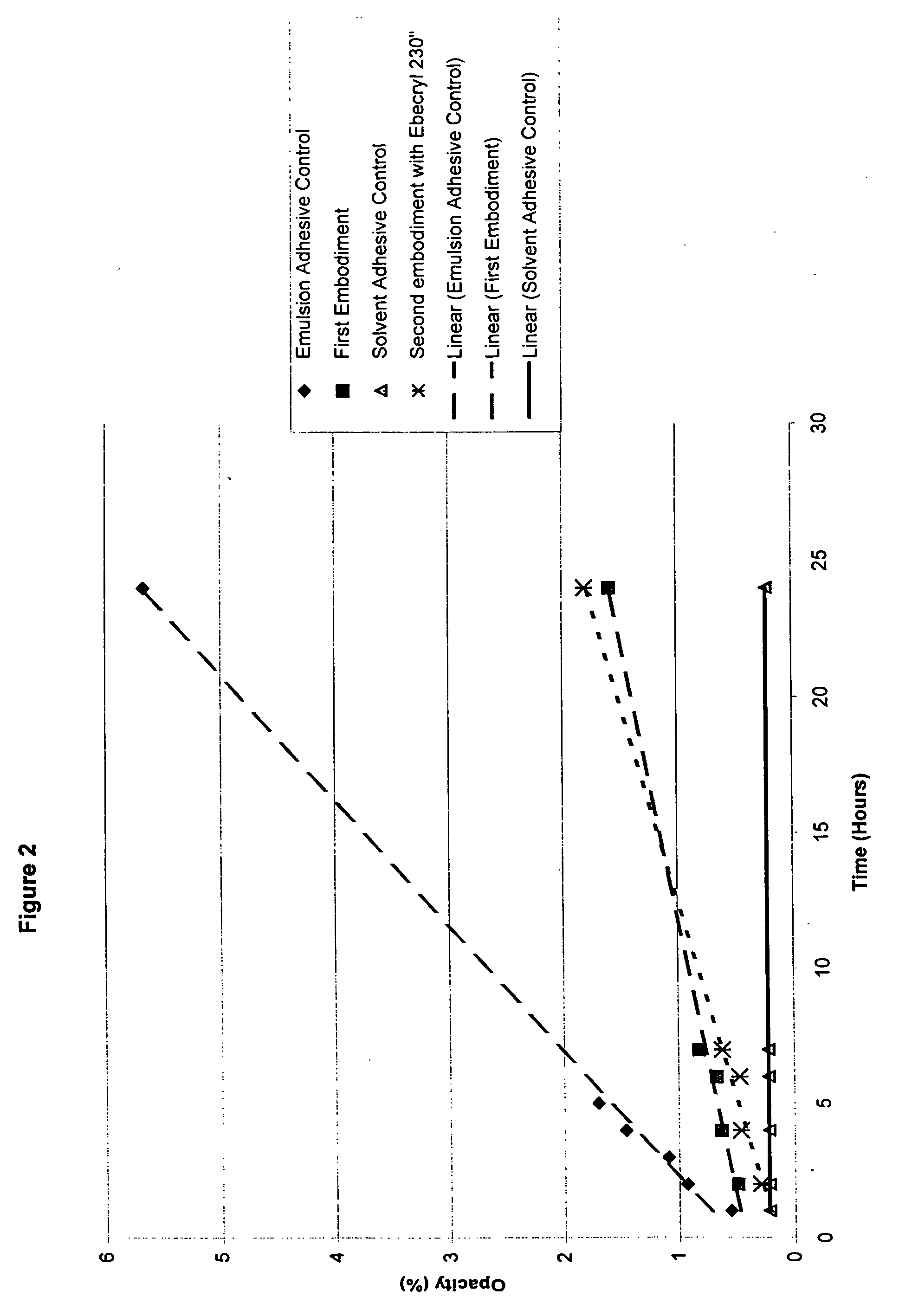
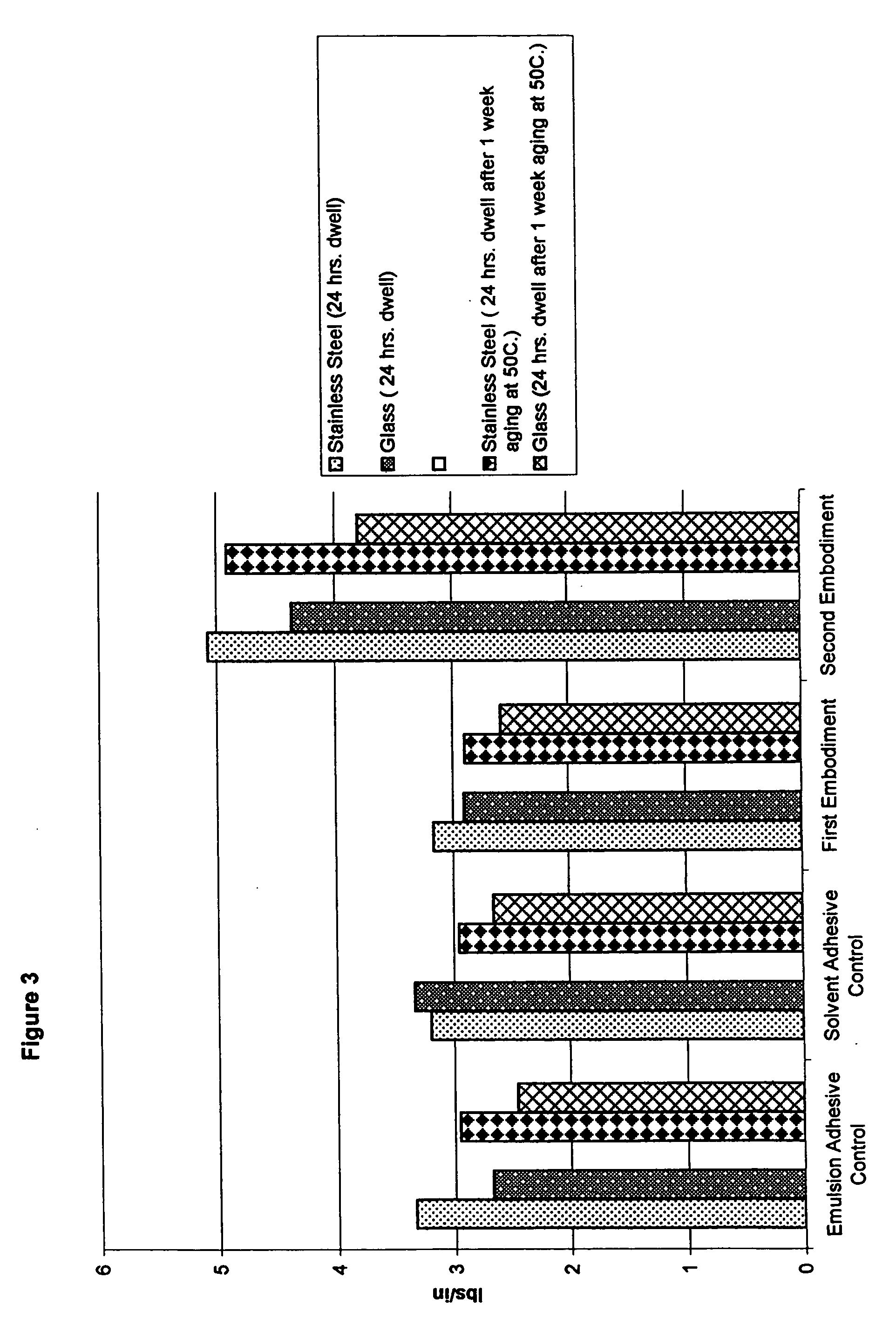
Water-whitening resistant pressure-sensitive adhesive
InactiveUS20050176876A1Improve abilitiesImprove propertiesEster polymer adhesivesEmulsion paintsPolymer scienceMeth-
Pressure-sensitive adhesive compositions that resist water-whitening are provided. The compositions comprise emulsion copolymers formed from a plurality of monomers that includes a plurality of (meth)acrylic monomers, at least one trifluoroalkyl(meth)acrylate monomer, and at least one alkylimidazolidone (meth)acrylate monomer. Preferably, the (meth)acrylic monomers comprise a plurality of soft monomers, at least one hard monomer and at least one acid monomer. The plurality of monomers may further include at least one aliphatic urethane di(meth)acrylate, an oligomer. The pressure-sensitive adhesive composition also comprises a surfactant system including at least one surfactant.
Owner:AVERY DENNISON CORP
Amino acid cleansing cream and preparation method thereof
ActiveCN102327198AImprove stabilityNo phototoxicityCosmetic preparationsToilet preparationsBetaineCocoyl glutamate
The invention discloses an amino acid cleansing cream and a preparation method thereof. The amino acid cleansing cream comprises the following components in percent by weight: 3-8 percent of glycerol, 0.05-0.2 percent of hydroxyethylcellulose, 5-13 percent of propylene glycol, 25-30 percent of sodium lauroyl glutamate, 5-8 percent of sodium cocoyl glutamate, 0.5-1.5 percent of ethal-octadeca-composite fatty alcohol, 0-2 percent of ethylene glycol distearate, 0.2-0.6 percent of cetearyl alcohol ether (20), 0.5-1.5 percent of cocoaminopropyl dimethyl betaine, 0.5-1.5 percent of cetearyl alcoholether-60 myristyl glycol, 1-3 percent of acrylate / polyoxyethylene-20 stearin alcohol ether methacrylate copolymer, 0.4-0.8 percent of citric acid, 0.1-0.2 percent of diazolidiny urea, 0.10-0.15 percent of methyl phydroxybenzoate and the balance of deionized water. the amino acid cleansing cream has better stability and solid content of sodium lauroyl glutamate of more than or equal to 25 percent,and can create a comfortable and clear microenvironment for healthy growth and metabolism for skin cells on the face.
Owner:SHENZHEN GENE BIOLOGICAL TECH
Use of a catalyst system comprising nickel, palladium, or platinum and imidazoline-2-ylidene or imidazolidine-2-ylidene in kumada coupling reactions
InactiveUS6369265B1Good yieldEliminate needOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsLiquid mediumGrignard reagent
This invention provides a process for conducting Kumada coupling reactions. The processes of the present invention make use of N-heterocyclic carbenes as ancillary ligands in Kumada couplings of aryl halides. A Kumada coupling can be carried out by mixing, in a liquid medium, at least one aryl halide, wherein the aryl halide has, directly bonded to the aromatic ring(s), at least one halogen atom selected from the group consisting of a chlorine atom, a bromine atom, and an iodine atom; at least one Grignard reagent; at least one metal compound comprising at least one metal atom selected from nickel, palladium, and platinum, wherein the formal oxidation state of the metal is zero or two; and at least one N-heterocyclic carbene. One preferred type of N-heterocyclic carbene is an imidazoline-2-ylidene of the formulawherein R1 and R2 are each, independently, alkyl or aryl groups having at least 3 carbon atoms, R3 and R4 are each, independently, a hydrogen atom, a halogen atom, or a hydrocarbyl group. Homocoupling of aryl pseudohalides is also feasible using the processes of this invention.
Owner:UNIV OF NEW ORLEANS RES TECH FOUND
Use of catalyst system comprising nickel, palladium, or platinum and imidazoline-2-ylidene or imidazolidine-2-ylidene in amination reactions
InactiveUS6403802B1High yieldEliminate needGroup 5/15 element organic compoundsCarboxylic acid esters preparationLiquid mediumOxidation state
This invention provides a process for conducting amination reactions. The processes of the present invention make use of N-heterocyclic carbenes as ancillary ligands in aminations of aryl halides and aryl pseudohalides. An amination can be carried out by mixing, in a liquid medium, at least one strong base; at least one aryl halide or aryl pseudohalide in which all substituents are other than amino groups, wherein the aryl halide has, directly bonded to the aromatic ring(s), at least one halogen atom selected from the group consisting of a chlorine atom, a bromine atom, and an iodine atom; at least one primary amine and / or at least one secondary amine; at least one metal compound comprising at least one metal atom selected from nickel, palladium, and platinum, wherein the formal oxidation state of the metal is zero or two; and at least one N-heterocyclic carbene. One preferred type of N-heterocyclic carbene is an imidazoline-2-ylidene of the formulawherein R1 and R2 are each, independently, alkyl or aryl groups having at least 3 carbon atoms, R3 and R4 are each, independently, a hydrogen atom, a halogen atom, or a hydrocarbyl group.
Owner:UNIV OF NEW ORLEANS RES TECH FOUND
Novel imidazolidine derivative and use thereof
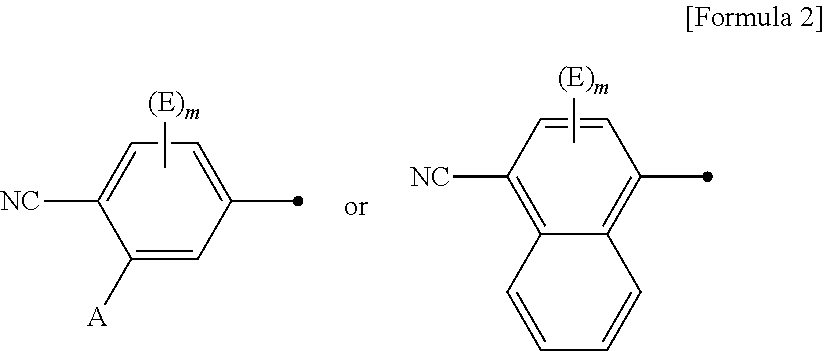
According to the present invention, a compound represented by formula (I):wherein Q is:A is a hydrogen atom, a halogen atom, —ORa or a C1-4 alkyl group which may be substituted by one or more halogen atoms; E is independently selected from a C1-6 alkyl group; m is selected from integers from 0 to 3; R2 and R3 are independently selected from a C1-6 alkyl group; X1 and X2 are independently selected from O and S; Y is selected from an arylene group and a divalent 5- or 6-membered monocyclic or 8- to 10-membered condensed heterocyclic group, wherein the arylene group and the heterocyclic group may be substituted by 1 to 3 substituents independently selected from E1; E1 is independently selected from a hydroxyl group, a halogen atom, a C1-4 alkyl group, a cyano group, a C1-4 alkoky group, a carbamoyl group, a C1-4 alkylcarbamoyl group, a di(C1-4 alkyl)carbamoyl group, an amino group, a C1-4 alkylamino group, a di(C1-4 alkylamino group, a sulfamoyl group, a C1-4 alkylsulfamoyl group and a di(C1-4 alkyl)sulfamoyl group; Z is —CON(—Ra)—, —CO—, —COO—, —NRa—C(═NH)NRb—, —NRa—C(═N—CN)NRb—, —N(—Ra)COO—, —C(═NH)—, —SO2—, —SO2N(—Ra)—, —SO2NR1—, —N(—Ra)CO—, —N(—Ra)CON(—Rb)—, —N(COR1)CO—, —N(—Ra)SO2—, —N(SO2R1)SO2—, —N(—Ra)— or —N(—Ra)SO2N(—Rb)—; R1 is independently a hydrogen atom, a hydroxyl group, a C1-6 alkyl group which may be substituted by one or more substituents, a heterocyclic group which may be substituted by one or more substituents, an aryl group which may be substituted by one or more substituents, a C3-8 cycloalkyl group which may be substituted by one or more substituents or a C3-8 cycloalkenyl group which may be substituted by one or more substituents, or a salt, prodrug or solvate thereof is provided. Furthermore, a pharmaceutical composition containing the compound, and the like are also provided.
Owner:CHUGAI PHARMA CO LTD
Flame-retardant steel surface coating material and preparation method thereof
InactiveCN104673042AImprove high temperature resistanceHigh mechanical strengthFireproof paintsAntifouling/underwater paintsAntioxidantAnti bacteria
The invention discloses a flame-retardant steel surface coating material and a preparation method thereof. The flame-retardant steel surface coating material is prepared from the following components in parts by weight: 35-48 parts of polyacrylamide, 5-9 parts of zinc oxide, 0.05-0.2 part of ammonium polyphosphate, 0.05-0.1 part of dibutyltin maleate, 0.03-0.1 part of diazolidinyl urea, 0.02-0.04 part of silane coupling agents, 0.01-0.2 part of antioxidants and 15-20 parts of 1,2-pentanediol. The invention also provides the preparation method of the flame-retardant steel surface coating material. The flame-retardant steel surface coating material prepared through the method disclosed by the invention not only has good flame-retardant property, but also has good scraping resistant property and antibacterial property. The flame-retardant steel surface coating material prepared through the method disclosed by the invention can be widely applied to the surfaces of a household appliance, an instrument, a meter, air purifying equipment and water purifying equipment.
Owner:SUZHOU KANGHUA PURIFYING SYST ENG
Synthetic method of imidacloprid
The invention provides a synthetic method of imidacloprid, which comprises the synthetic steps of (1) sequentially adding an amine substance, 2-chlorine-5-chlorine picoline and a solvent into a reactor, conducting heating reflux for 2h, cooling, obtaining a catalyst solution, (2) sequentially adding a solvent, 2-nitryl imidogen imidazolidine and 1 / 5 alkali metal hydroxide into the catalyst solution, stirring, heating to 45 DEG C for heat preservation; (3) dropwise adding a solvent solution of 2-chlorine-5-chlorine picoline into reaction liquid for 30min each time and for four times totally after the heat preservation is over, adding 1 / 5 alkali metal hydroxide into the reaction liquid after dropwise adding the solvent solution each time, conducting heat preservation reaction for 1-2h after finishing the adding, and (4) conducting water washing for three times after the reaction is over, distilling the solvent out, crystallizing at 5 DEG C below zero, conducting suction filtration, drying, and obtaining a crude product of imidacloprid. According to the synthetic method, the reaction time is short; by-products are few; the purity of the crude product of imidacloprid reaches 98%; and a yield reaches 95.3%.
Owner:SHANDONG UNITED PESTICIDE IND CO LTD
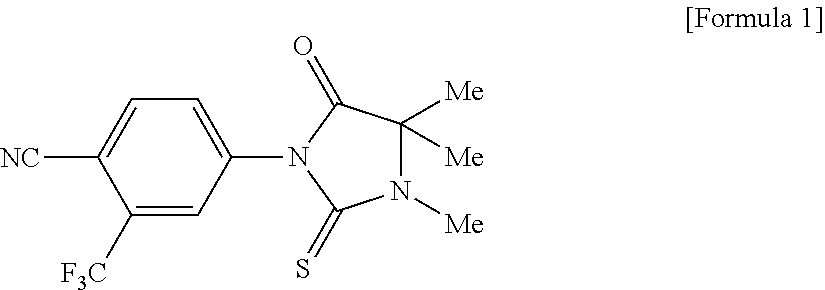
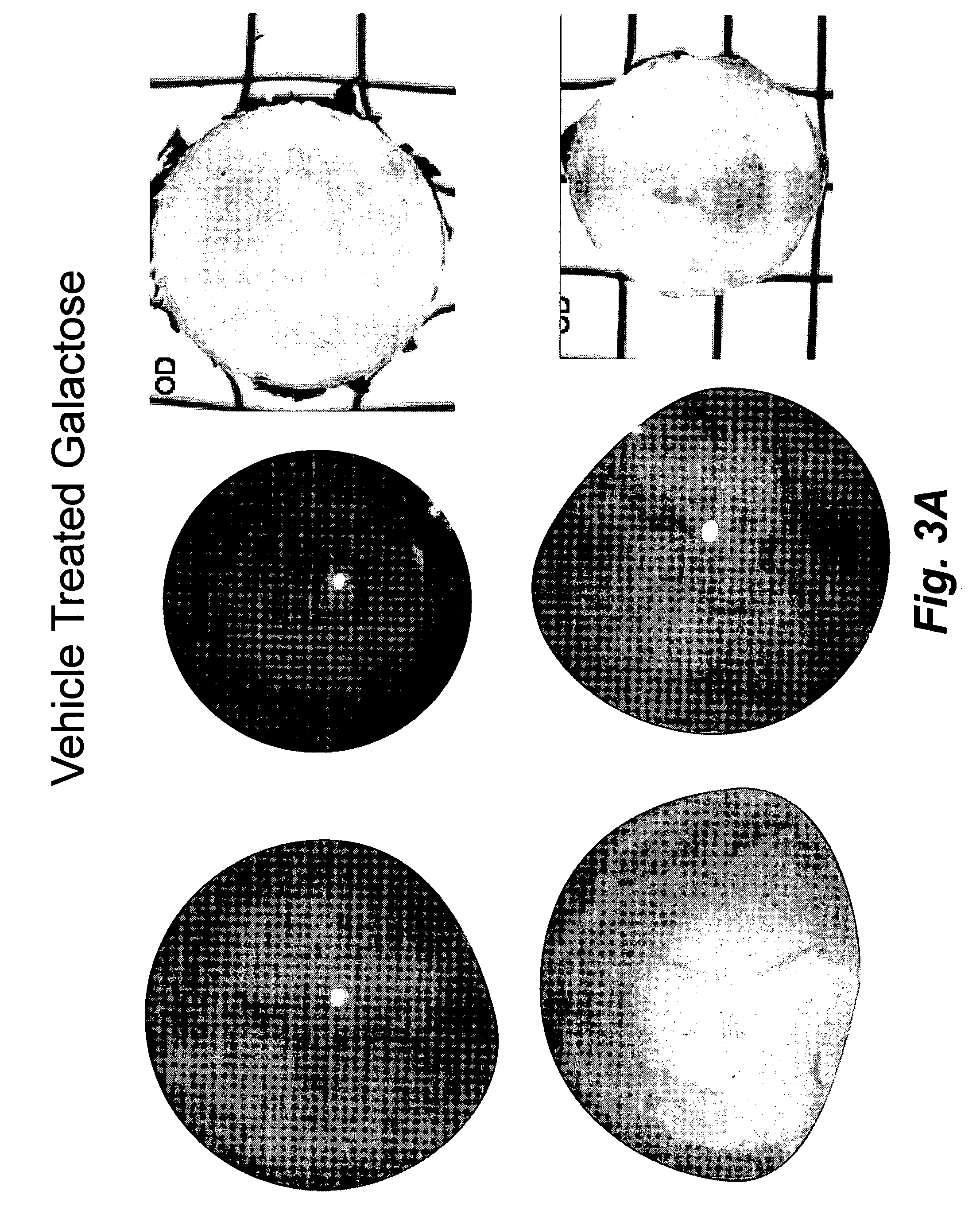
Topical treatment of cataracts in dogs
ActiveUS8158667B2Improved stereospecific synthesis of R-methylReduces number of stageBiocideOrganic active ingredientsDiabetic retinopathyGlycerol
Owner:BOARD OF RGT UNIV OF NEBRASKA
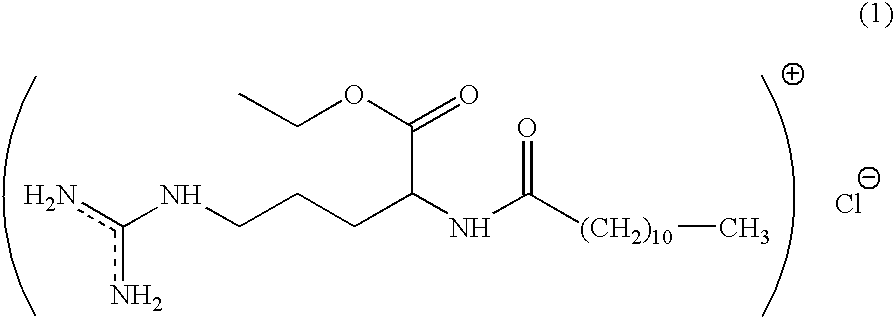
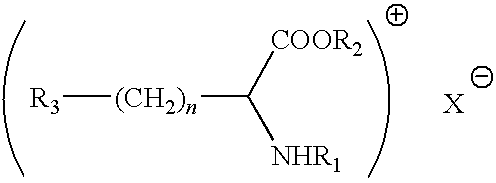
Preservative systems and their use in cosmetic preparations
The invention relates to preservative systems which are particularly suitable in cosmetic and dermatological preparations. The preservative system which comprises a cationic surfactant, derived from the condensation of fatty acids and esterified dibasic amino acids, having the formula (I) where: X− is Br−, Cl−, or HSO4 R1: is a straight alkyl chain from an acid or saturated fatty hydroxy acid from 8 to 14 atoms of carbon bonded to the α-amino acid group through amidic bond. R2: is a straight or branched alkyl chain from 1 to 18 carbon atoms or aromatic. R3: is: (II) where n can be from 0 to 4, is combined with at least one other ionic or non-ionic preservative agent, whereby the combination displays a synergetic activity. A preservative system, wherein the cationic surfactant preservative derived from the condensation of fatty acids and esterified dibasic amino acid is LAE is particularly preferred. The other ionic or non ionic preservative agent is preferably at least one component selected from the group consisting of 2-bromo-2-nitro-1,3-propanediol (bronopol), parbens, imidazolidinyl urea, phenoxyethanol, DMDM hydantoin, 2-methyl-5-chloro-3,4-isothiazolinone / 2-methyl-3,4-isothiazolinone and quaternium-15.
Owner:LAB MIRET
Process for producing methionine
ActiveCN101735125AEfficient recyclingEasy to manufactureOrganic compound preparationSulfide preparationAlcoholPotassium
A process for producing methionine advantageously in view of cost, while efficiently recovering useful components, is provided. The present invention relates to a process for producing methionine, comprising the following steps (1), (2), (3) and (4): (1) a reaction step of hydrolyzing 5-[2-(methylthio)ethyl]imidazolidine-2,4-dione in the presence of a basic potassium compound; (2) a first crystallization step of introducing carbon dioxide into the reaction solution obtained in the step (1) to thereby precipitate methionine, and separating the resulting slurry into a precipitate and a mother liquor; (3) a second crystallization step of concentrating the mother liquor obtained in the step (2), mixing the resulting concentrate with a lower alcohol, introducing carbon dioxide into the resulting mixture to thereby precipitate methionine and potassium hydrogencarbonate, and separating the resulting slurry into a precipitate and a mother liquor; and (4) a heating step of concentrating the mother liquor obtained in the step (3), treating the resulting concentrate by heating at a temperature of from 150 to 200 DEG C, and recycling the treated solution for use in the step (3).
Owner:SUMITOMO CHEM CO LTD
Process for producing methionine
InactiveUS20100004486A1Easy to separateEfficient executionThiol preparationOrganic compound preparationPotassiumKetone
The present invention provides a process for producing methionine which is advantageous from the viewpoints of cost and wastewater disposal, and which comprises the following steps (1) to (5):(1) a reaction step comprising hydrolysis of 5-[2-(methylthio)ethyl]imidazolidine-2,4-dione in the presence of a basic potassium compound;(2) a first crystallization step comprising introduction of carbon dioxide into the reaction solution obtained in the step (1) to precipitate methionine, and separation of the resultant slurry into a precipitate and a mother liquor;(3) a second crystallization step comprising concentration of the mother liquor obtained in the step (2), mixing with a lower alcohol, introduction of carbon dioxide into the mixture to precipitate methionine and potassium hydrogen carbonate, and separation of the resultant slurry into a precipitate and a mother liquor;(4) a heating step comprising concentration of the mother liquor obtained in the step (3) followed by a heating treatment at 150 to 200° C.; and(5) a third crystallization step comprising introduction of carbon dioxide into the heated mother liquor obtained in the step (4) to precipitate methionine and potassium hydrogen carbonate, and separation of the resultant slurry into a precipitate and a mother liquor.
Owner:SUMITOMO CHEM CO LTD
High-temperature-resistant desulfurizer for oil-gas field and preparation method of desulfurizer
ActiveCN107418641AImprove desulfurization efficiencyReduced fouling tendencyGaseous fuelsEnvironmental resistanceSulfate
The invention provides a high-temperature-resistant desulfurizer for the oil-gas field and a preparation method of the desulfurizer. 30%-60% of a triazine compound, 5%-20% of organic phosphonic acid, 5%-20% of heterocyclic organic amine, 5%-20% of water-soluble imidazolidine and the balance of water are weighed firstly, wherein the sum of mass percentages of all the components is 100%; the triazine compound is added to water and stirred uniformly, and a mixture B is obtained; heterocyclic organic amine, water-soluble imidazolidine and organic phosphonic acid are added to the mixture B sequentially, the mixture is stirred uniformly, and the high-temperature-resistant desulfurizer for the oil-gas field is obtained. The prepared desulfurizer has high desulfurization efficiency under the high-temperature condition of 90 DEG C; the product has certain scale and corrosion inhibition and sterilization performance; the prepared desulfurizer has the pH value being 7-8, and the scaling trend of carbonate and sulfate in the highly mineralized water environment can be delayed; the whole synthesis process adopts simple conditions and is easy to operate, low in cost, non-toxic, environment-friendly and suitable for industrial production.
Owner:陕西日新石油化工有限公司
Nitroimidazoline derivatives and preparation method thereof and application thereof
InactiveCN101830853AIncrease virulenceThe synthesis method is simpleBiocideOrganic chemistryBenzoxazoleImidacloprid
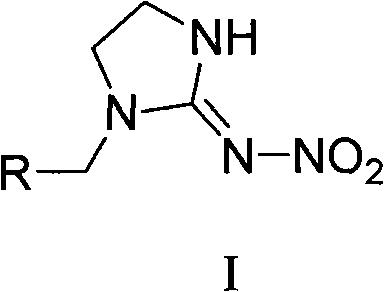
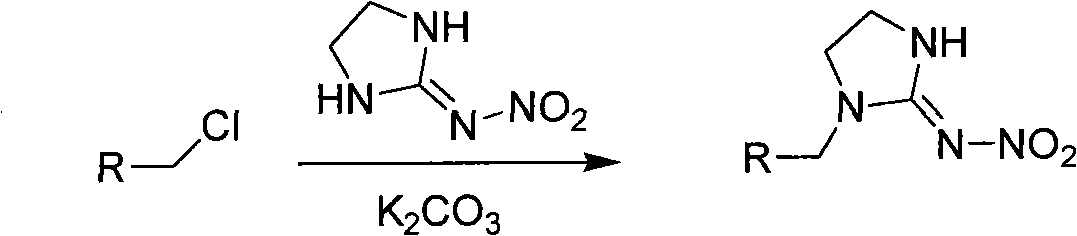
The invention relates to nitroimidazoline derivatives. The nitroimidazoline derivatives have a structural formula shown in a formula I, wherein R is substituted benzene, naphthalene, benzoxazole or benzimidazole. The preparation method comprises that different substituted chloromethylene compounds, N-nitroimidazoline, potassium carbonate and solvent are reacted to form the nitroimidazoline derivatives. The nitroimidazoline derivatives have the advantages that: 1) the synthetic method for the nitroimidazoline derivative compounds is simple; and 2) the nitroimidazoline derivative compounds have a structure different from that of imidacloprid and have high insecticidal activity, the structure of the compounds does not comprise pyridine rings, and the compounds have wide development prospect.
Owner:WUHAN INSTITUTE OF TECHNOLOGY
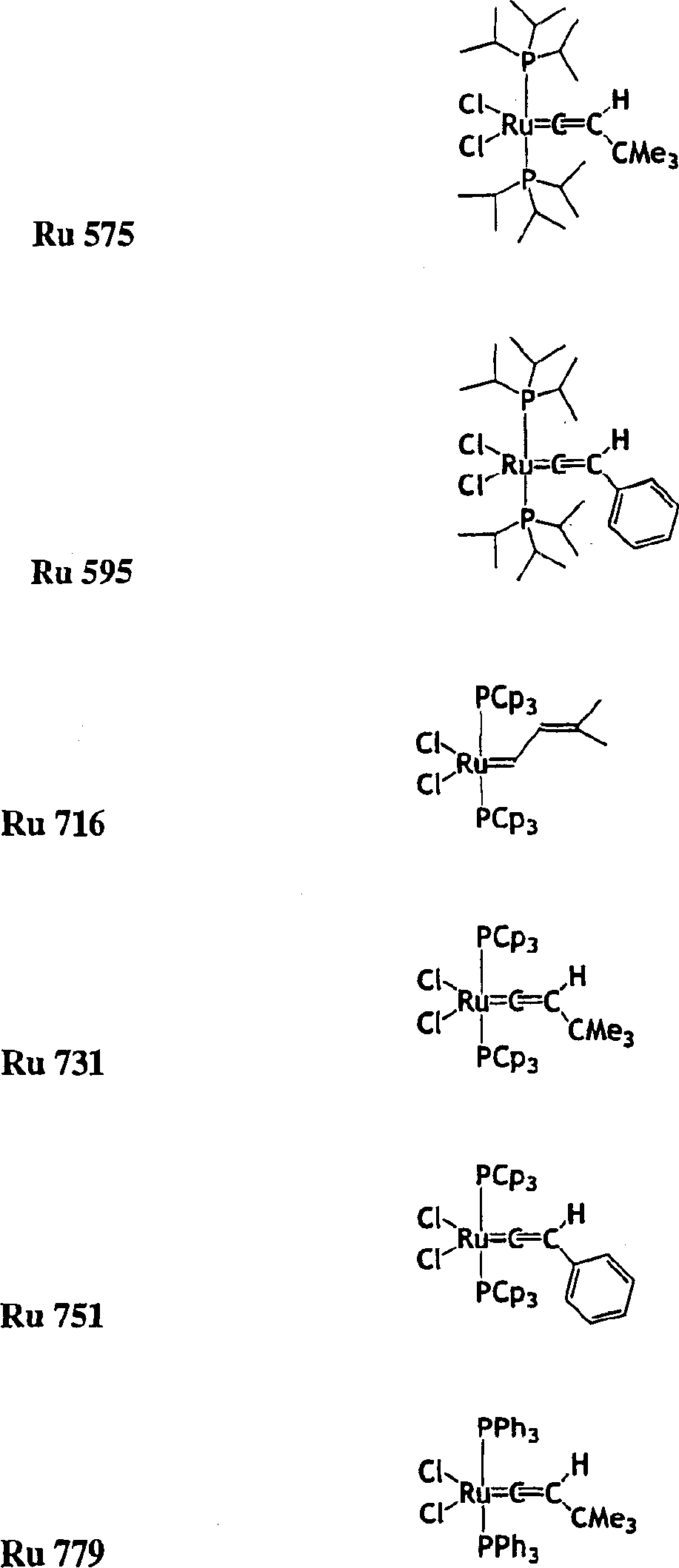
High activity metal carbene metathesis catalysts generated using thermally activated N-heterocyclic carbene precursor
InactiveCN1511064ARuthenium organic compoundsOrganic-compounds/hydrides/coordination-complexes catalystsCarbeneChloroform
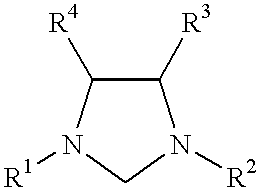
The invention provides a method of making a ruthenium or osmium carbene catalyst having an imidazolidine in the presence of an olefin with the application of energy. The resulting metal compound has two carbene ligands, one from the original carbene, the other from the imidazolidine. The imidazolidine has bulky protecting groups in the two positions adjacent to the carbene carbon. The method of making the imidazoline from the salt in the presence of a base and chloroform is also disclosed.
Owner:CALIFORNIA INST OF TECH +1
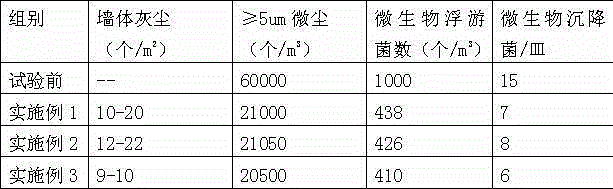
Coating and preparation method thereof
ActiveCN105199587AImprove cleanlinessAvoid inconvenienceAntifouling/underwater paintsPaints with biocidesRare earthToxic material
The invention discloses a coating and a preparation method thereof. The coating is prepared from raw materials in parts by mass as follows: 20-30 parts of waterborne resin, 10-15 parts of a polyurethane emulsion, 10-15 parts of diatomaceous earth, 8-15 parts of rare earth, 3-8 parts of polyacrylamide, 2-4 parts of imidazolidine, 0.5-1 part of hydroxymethyl cellulose, 1-3 parts of a nano-assistant, 5-10 parts of an adsorption auxiliary, 1-3 parts of a bactericide, 0.5-1 part of a film forming agent, 0.3-1 part of an antifoaming agent, 0.5-1 parts of a leveling agent, 0.2-0.5 parts of a wetting agent, 0.5-1 part of a dispersion agent, 0.4-1 part of a thickening agent, 2-4 parts of titanium dioxide and 20-30 parts of deionized water. The invention further discloses a preparation method. The coating and the preparation method have the benefits as follows: the prepared coating can prevent dust and keep clean after being applied to a wall, the inconvenience caused by dust accumulation is avoided, long-time cleanness is realized, and the toxic substances in air are purified.
Owner:HEBEI CHENYANG INDAL & TRADE GROUP CO LTD
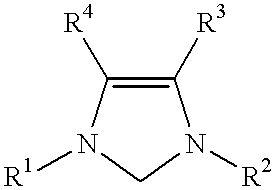
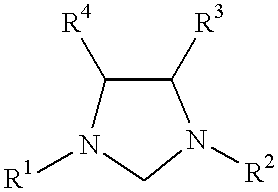
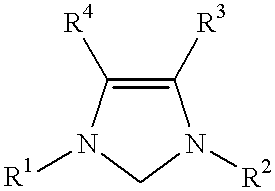
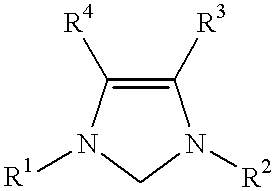
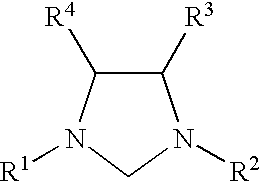
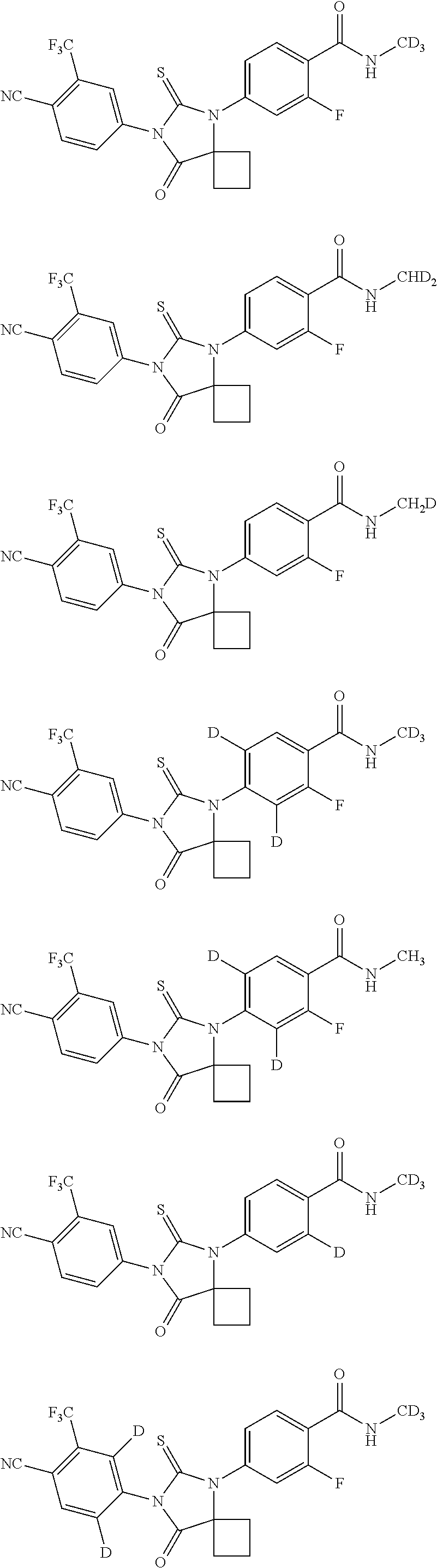
Novel Spiro [Imidazolidine-4, 3' -Indole] 2, 2', 5' (1H) Triones for Treatment of Conditions Associated with Vanilloid Receptor 1
Owner:ASTRAZENECA AB
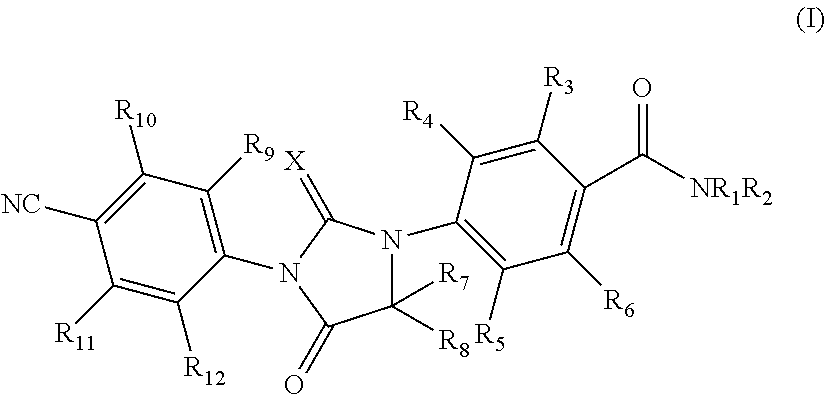
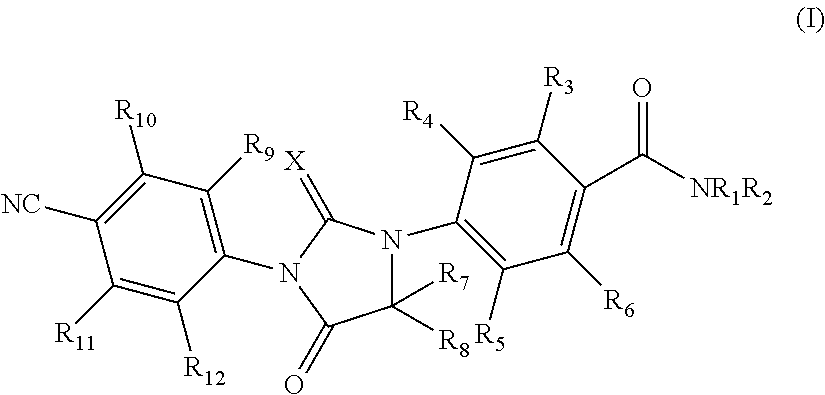
Imidazolidinedione compounds and their uses
Provided are imidazolidinedione compounds of formula (I), processes for preparation, uses and pharmaceutically compositions thereof. Said imidazolidinedione compounds posses androgen receptor antagonist activity and can be used for preventing and treating diseases and disorders related to androgen receptor, such as prostate cancer, alopecia, hair regeneration, acne and adolescent acne.
Owner:HINOVA PHARM INC
Air purifying liquid and preparation method thereof
The invention relates to an air purifying liquid and a preparation method thereof. The air purifying liquid comprises the raw materials in parts by weight: 1 to 50 parts by weight of glycerol, 1 to 15 parts by weight of polyethylene glycol, 0.5 to 5 parts by weight of dendrimers, 0.1 to 5 parts by weight of an imidazole cation liquid, 0.5 to 10 parts by weight of imidazolizone, 0.1 to 5 parts by weight of a pH regulator, and the balance deionized water. The preparation method comprises the steps: adding glycerol and polyethylene glycol into deionized water, and stirring until the solution is uniform and transparent; adding the dendrimers in the formed uniform and transparent solution, and stirring until the solution is uniform and transparent; adding the pH regulator to adjust the solution pH to 6.5-8.5, and stirring uniformly; and adding the imidazole cation liquid component and imidazolizone, and stirring uniformly, to obtain the purifying liquid finished product. The air purifying liquid is convenient to use: the air purifying liquid is injected into a spray bottle for operating, and is simple and convenient; the air purifying liquid is safe and reliable: the air purifying liquid is safe to human bodies and has no damage to objects; and the air purifying liquid has high purification efficiency: the air purifying liquid has the purification ability on formaldehyde and TVOCs more than or equal to 90%.
Owner:上海杉盛空气净化技术有限公司 +3
Full-biodegradable plastic composite modified material suitable for film bag and preparation method of full-biodegradable plastic composite modified material
ActiveCN113956623AGood mechanical propertiesImprove stiffnessBio-packagingButanedioic acidAdipic acid
The invention discloses a full-biodegradable plastic composite modified material suitable for a film bag. The full-biodegradable plastic composite modified material is mainly prepared from the following raw materials in percentage by mass: 25-30% of poly (butylene adipate-co-terephthalate); 25-30% of poly (butylene succinate); 10-15% of polylactic acid; 25-30% of calcium carbonate; and 4-6% of an auxiliary agent. The auxiliary agent is maleic anhydride, 2-imidazolidone and a copolymer of styrene and glycidyl acrylate. The composite modified material has excellent mechanical properties, high tensile strength and elongation at break, and good transparency, stiffness and printability. The invention further discloses a preparation method of the composite modified material. The method can prevent the degradable resin from being coked in the processing process, and the quality of the full-biodegradable plastic composite modified material is improved.
Owner:佛山粤晟达新材料有限公司
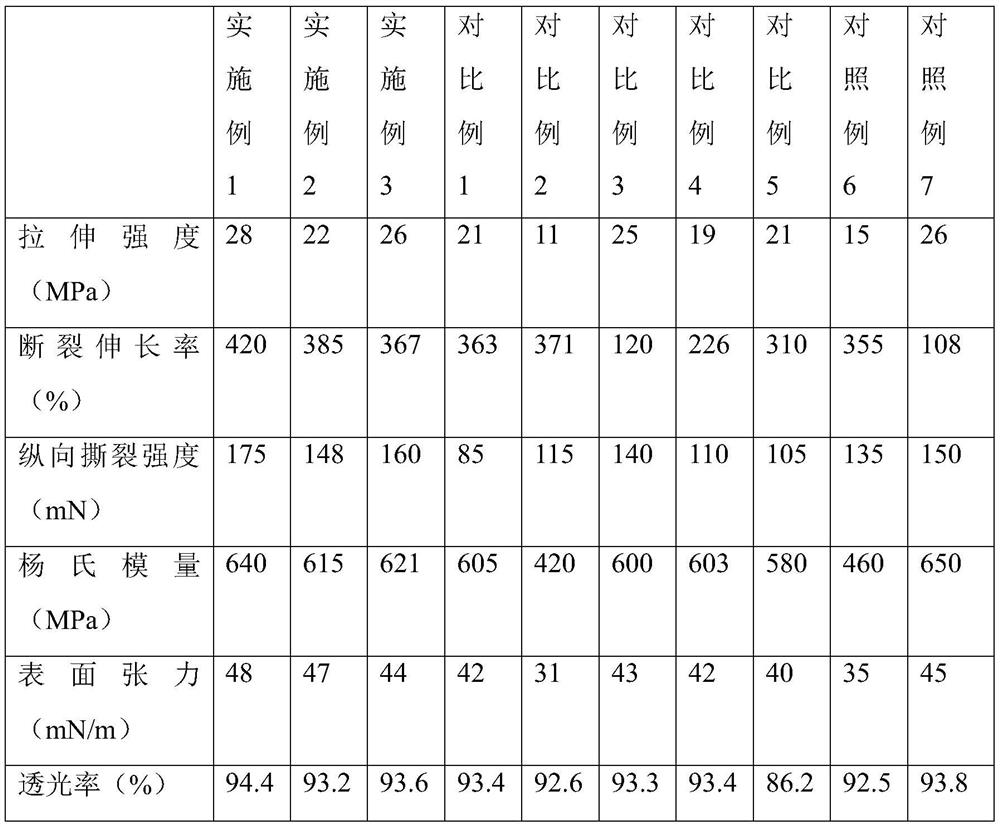
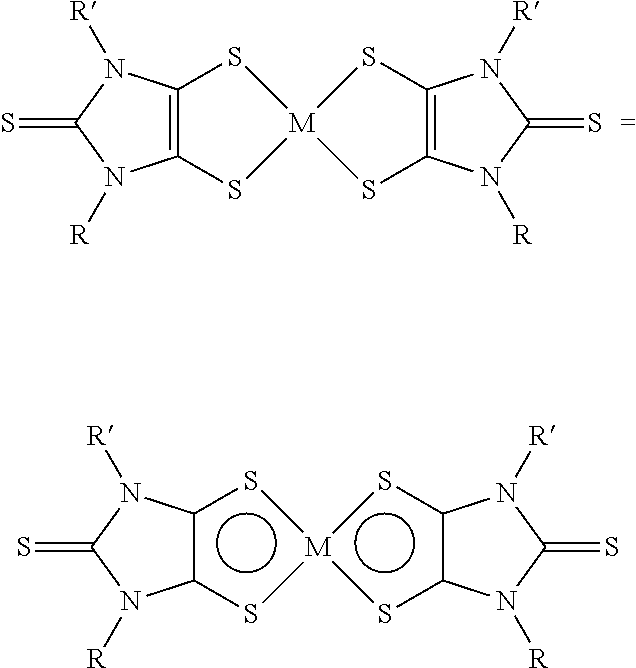
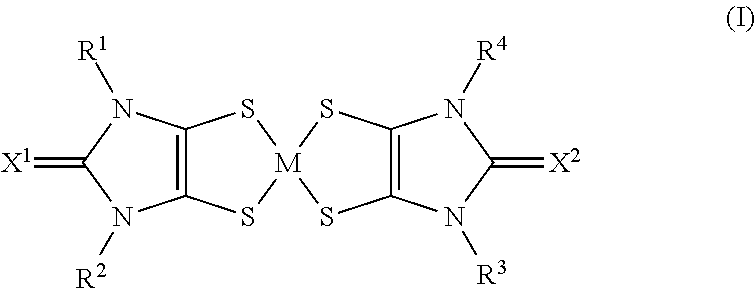
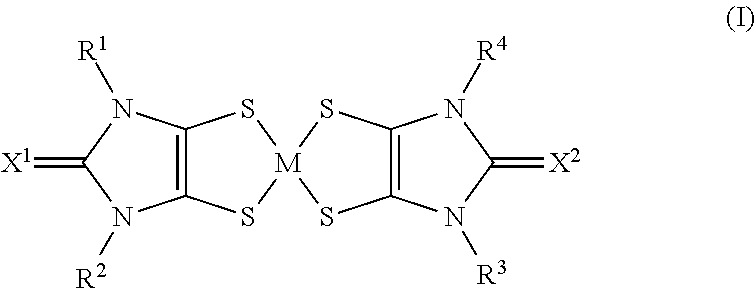
The use of aryl or heteroaryl substituted dithiolene metal complexes as ir absorbers
The present invention relates to the use of specific metal complexes of dithiolenes with aryl or heteroarylsubstituted imidazolidine-2-chalcogenone-4,5-dithione ligands as colourless IR absorbers.
Owner:BASF AG
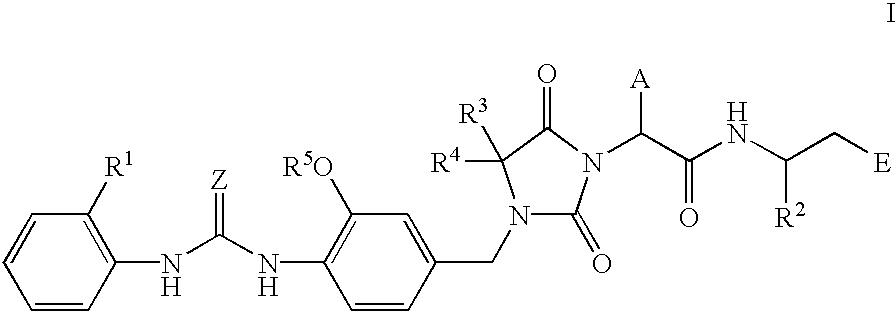
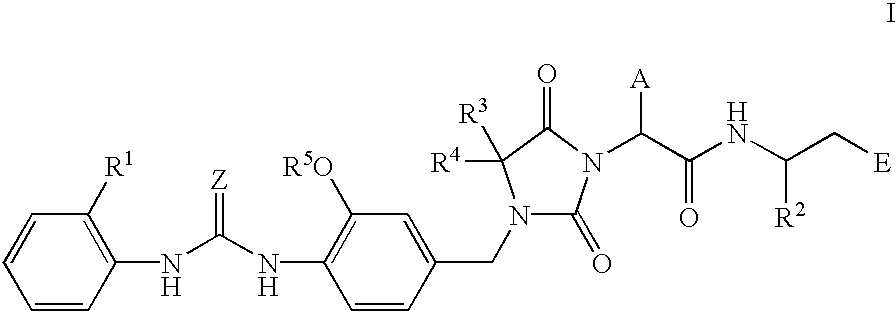
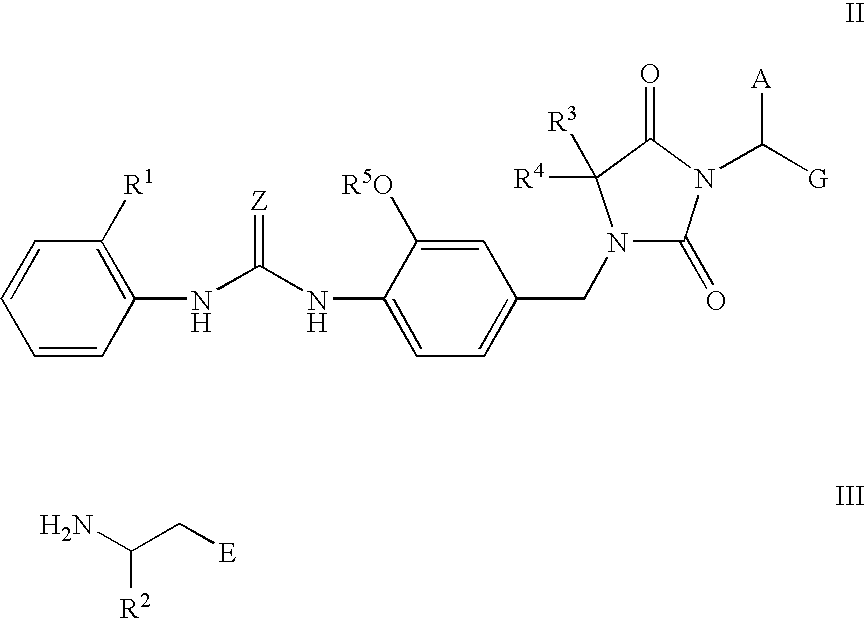
Imidazolidine derivatives, their preparation, their use and pharmaceutical preparations comprising them
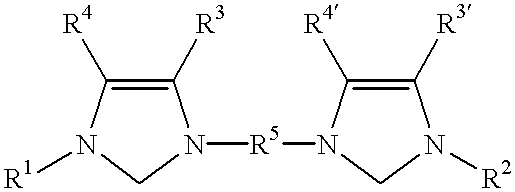
The present invention relates to novel imidazolidine derivatives of formula I,wherein A, E, Z, R<1>, R<2>, R<3>, R<4 >and R<5 >have the meanings indicated in the claims. The compounds of formula I are valuable pharmaceutical active compounds which are suitable, for example, for the treatment of inflammatory diseases, including rheumatoid arthritis, or allergic diseases. The compounds of formula I are inhibitors of the adhesion and migration of leukocytes and / or antagonists of the adhesion receptor VLA-4 belonging to the integrins group. They are generally suitable for the treatment of diseases which are caused by an undesired extent of leukocyte adhesion and / or leukocyte migration or are associated therewith or in which cell-cell or cell-matrix interactions which are based on the interactions of VLA-4 receptors with their ligands play a role. The invention furthermore relates to processes for the preparation of the compounds of formula I, their use and pharmaceutical preparations which contain compounds of formula I.
Owner:SANOFI AVENTIS DEUT GMBH
Synthetic method for semi-cucurbituril [6] with 2-imidazolidone and derivatives therefor as unit
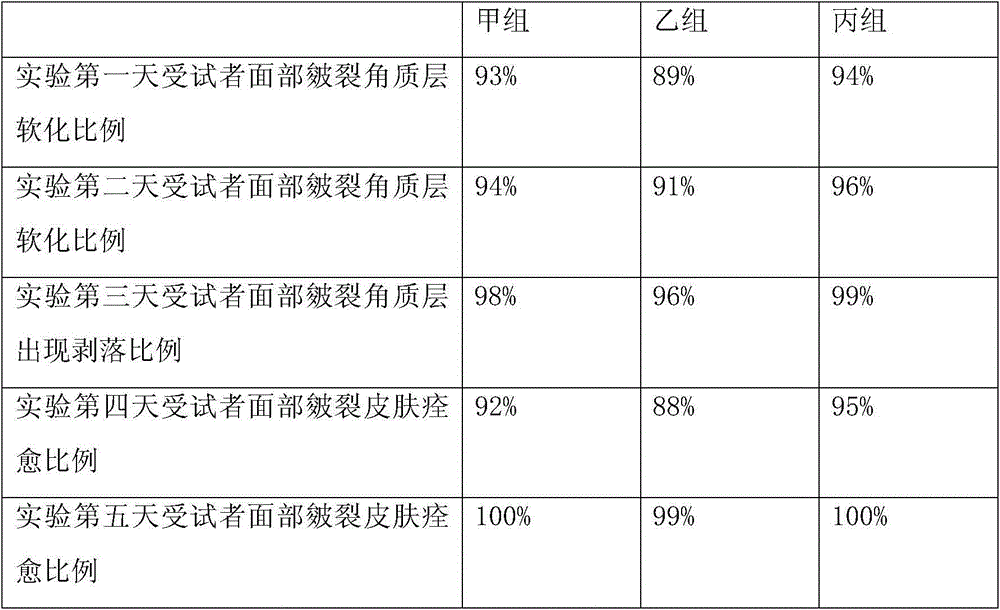
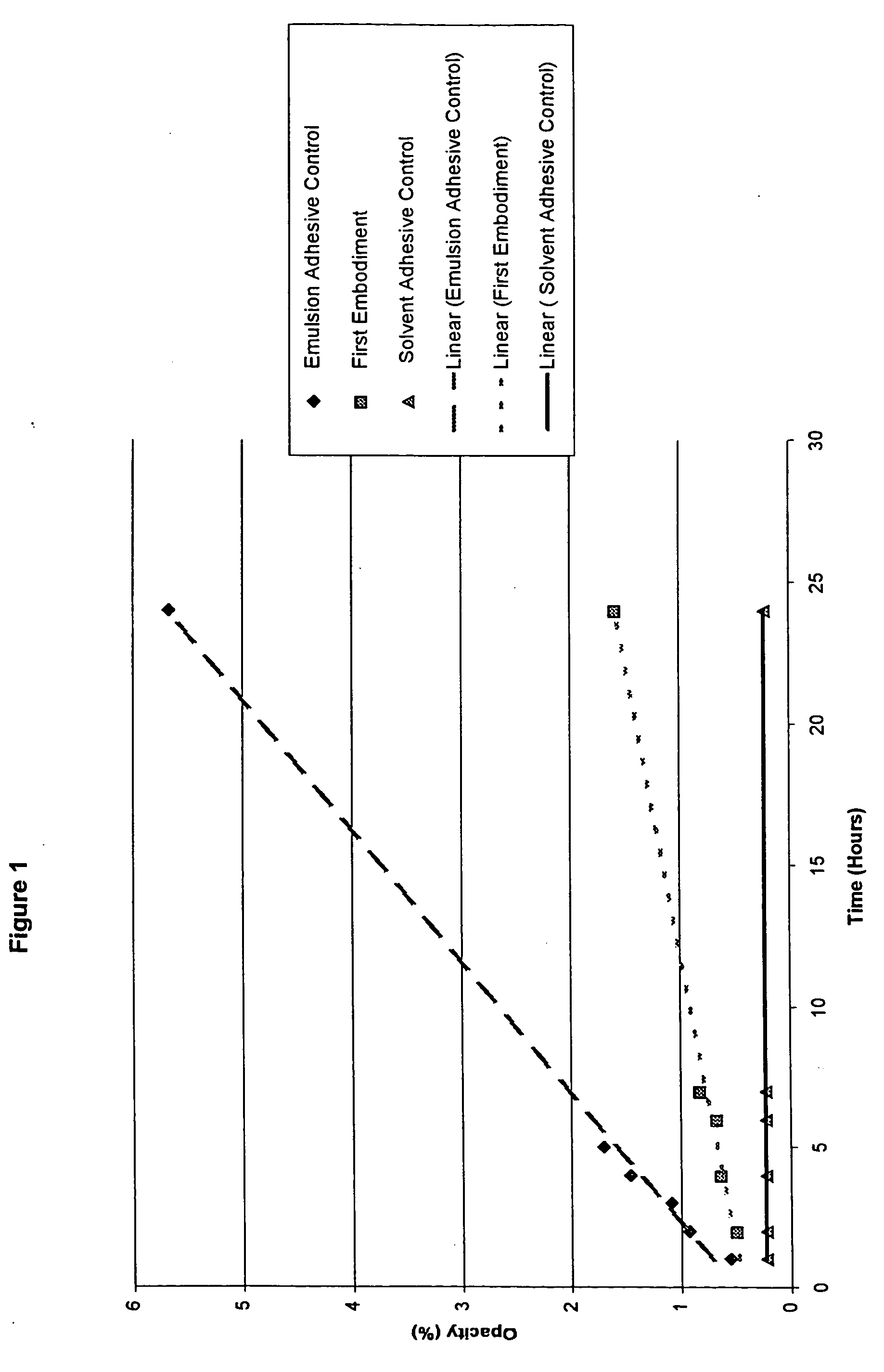
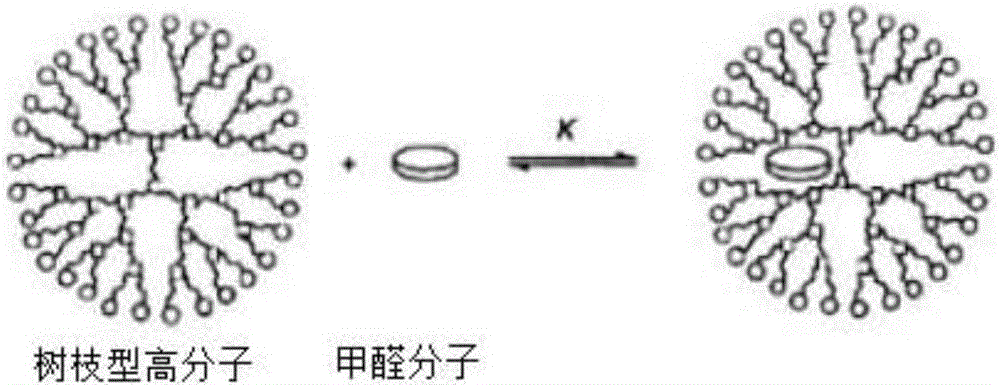
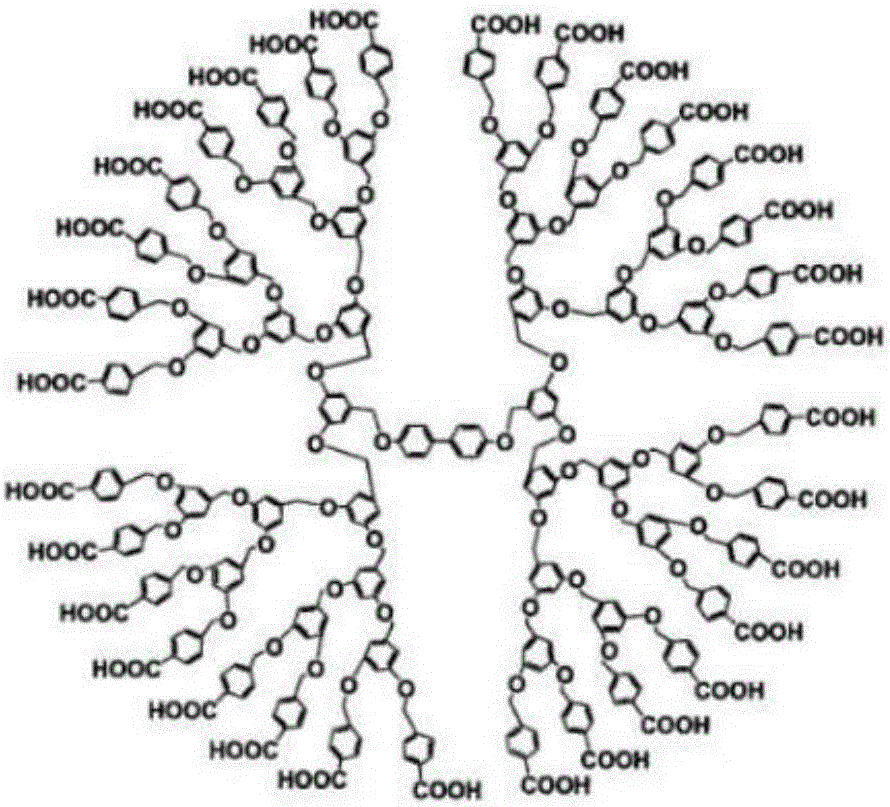
The invention provides a synthetic method for semi-cucurbituril [6] with 2-imidazolidone and derivatives therefor as a unit. Semi-cucurbituril is a six-membered polymerization ring with a structure similar to half structure of unit glycoluril for synthesis of cucurbituril as a unit, namely, with 2- imidazolidone as a unit, also named after semi-cucurbituril [6], and is an organic heterocyclic compound. The synthetic method employs 2-imidazolidone, derivatives therefor, and formaldehyde as raw materials. The raw materials are subjected to heating reflux for 4-24 h in an acidic aqueous solution system at the temperature of 40- 80 DEG C, and then subjected to condensation, filtration, isolation and purification to obtain semi-cucurbituril [6]. The material molar ratio of 2-imidazolidone and derivatives therefor to formaldehyde is 1:1-1.2. The catalytic acidic system can be inorganic acids or organic acids, with a concentration range from pH=2 to 5 mol / L. Semi-cucurbituril [6] can be synthetised with inorganic salts and ionic liquids as template agents. Semi-cucurbituril [6] with single component can be synthetised through control of conditions such as concentration of the reactants, acidity and temperature of the reaction system and the like.
Owner:GUANGXI UNIV
Imidazolidinedione derivatives for the treatment of diabetes and other diseases
The present invention relates to certain substituted heterocycles of Formula (I) which are useful in the treatment of diseases related to lipid and carbohydrate metabolism, such as type 2 diabetes, adipocyte differentiation, uncontrolled proliferation, such as lymphoma, Hodgkin's Disease, leukemia, breast cancer, prostate cancer or cancers in general; and inflammation, such as osteoarthritis, rheumatoid arthritis, Crohn's Disease or Inflammatory Bowel Disease.
Owner:ORTHO MCNEIL PHARM INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
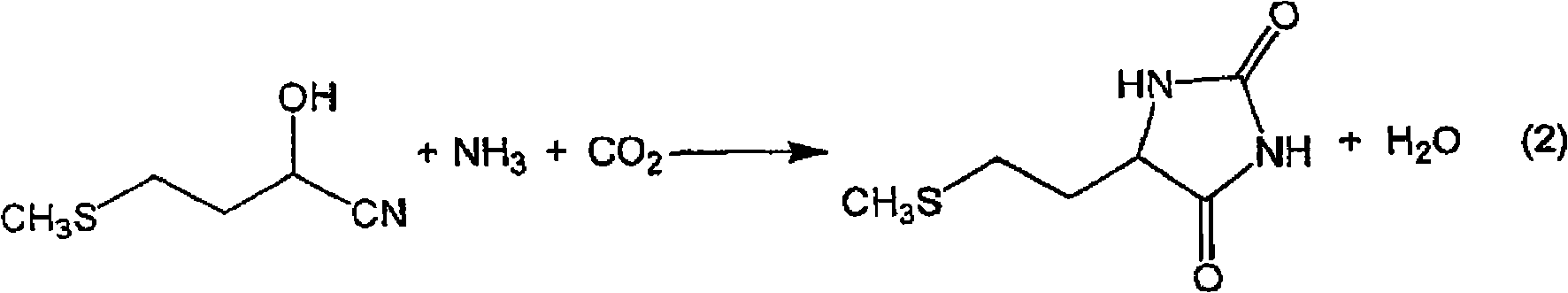
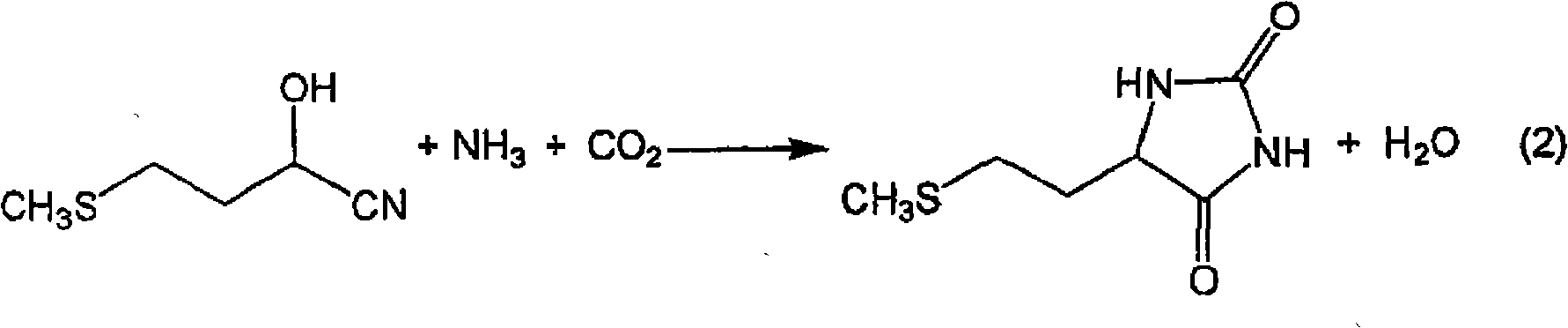
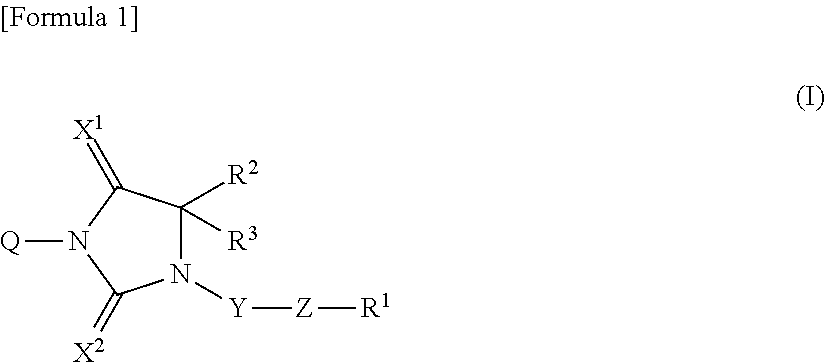
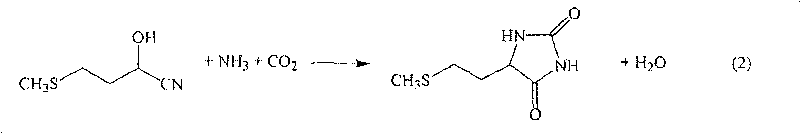


![Novel Spiro [Imidazolidine-4, 3' -Indole] 2, 2', 5' (1H) Triones for Treatment of Conditions Associated with Vanilloid Receptor 1 Novel Spiro [Imidazolidine-4, 3' -Indole] 2, 2', 5' (1H) Triones for Treatment of Conditions Associated with Vanilloid Receptor 1](https://images-eureka.patsnap.com/patent_img/b0053aaf-1960-4536-b02a-c40e8be4673c/US20090076049A1-20090319-C00001.png)
![Novel Spiro [Imidazolidine-4, 3' -Indole] 2, 2', 5' (1H) Triones for Treatment of Conditions Associated with Vanilloid Receptor 1 Novel Spiro [Imidazolidine-4, 3' -Indole] 2, 2', 5' (1H) Triones for Treatment of Conditions Associated with Vanilloid Receptor 1](https://images-eureka.patsnap.com/patent_img/b0053aaf-1960-4536-b02a-c40e8be4673c/US20090076049A1-20090319-C00002.png)
![Novel Spiro [Imidazolidine-4, 3' -Indole] 2, 2', 5' (1H) Triones for Treatment of Conditions Associated with Vanilloid Receptor 1 Novel Spiro [Imidazolidine-4, 3' -Indole] 2, 2', 5' (1H) Triones for Treatment of Conditions Associated with Vanilloid Receptor 1](https://images-eureka.patsnap.com/patent_img/b0053aaf-1960-4536-b02a-c40e8be4673c/US20090076049A1-20090319-C00003.png)

![Synthetic method for semi-cucurbituril [6] with 2-imidazolidone and derivatives therefor as unit Synthetic method for semi-cucurbituril [6] with 2-imidazolidone and derivatives therefor as unit](https://images-eureka.patsnap.com/patent_img/0f6ea800-e392-475a-96e4-9c1cdf4b3d17/HSA00000699541600011.png)
![Synthetic method for semi-cucurbituril [6] with 2-imidazolidone and derivatives therefor as unit Synthetic method for semi-cucurbituril [6] with 2-imidazolidone and derivatives therefor as unit](https://images-eureka.patsnap.com/patent_img/0f6ea800-e392-475a-96e4-9c1cdf4b3d17/HSA00000699541600012.png)
![Synthetic method for semi-cucurbituril [6] with 2-imidazolidone and derivatives therefor as unit Synthetic method for semi-cucurbituril [6] with 2-imidazolidone and derivatives therefor as unit](https://images-eureka.patsnap.com/patent_img/0f6ea800-e392-475a-96e4-9c1cdf4b3d17/HSA00000699541600013.png)