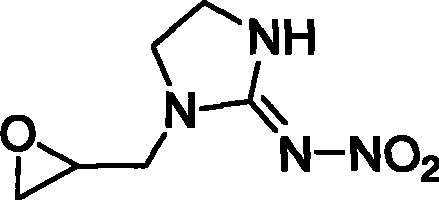
1-(1,2-epoxy propyl)-n-nitroimidazolene amine-2, preparation and use thereof
A technology of nitroimidazolidine and glycidyl ethylenediamine, applied to 1-(1,2-epoxypropyl)-N-nitroimidazolidine-2-ylamine and its preparation and application It can solve problems such as difficulty in governance and synthesis methods that have not yet been reported in the literature.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
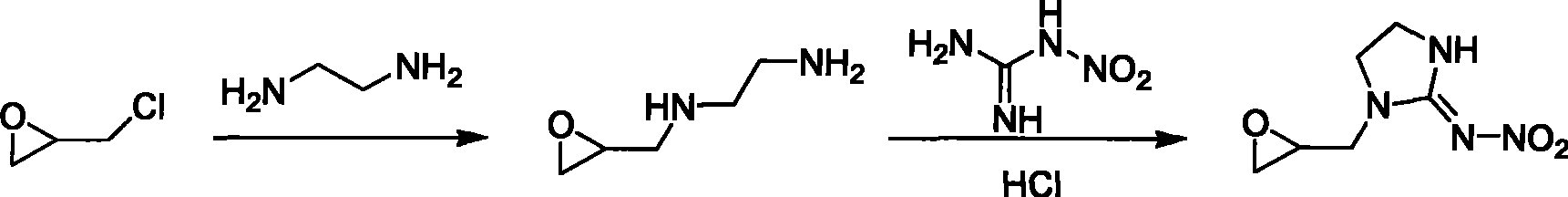
[0025] (1) synthesis of epoxypropyl ethylenediamine
[0026] Add 24g of ethylenediamine into a 250ml three-necked bottle, dissolve 37g of epichlorohydrin in 120ml of acetonitrile solvent, put it into a constant pressure titration funnel, add dropwise to the bottle while stirring, react at 45°C for 2.5h, cool, evaporate Go out acetonitrile and obtain epoxypropyl ethylenediamine;
[0027] (2) Synthesis of 1-(1,2-epoxypropyl)-N-nitroimidazolidin-2-ylamine
[0028] Dissolve 27.8g of epoxypropyl ethylenediamine and 15.6g of nitroguanidine in 200ml of water, slowly add 14.8g of concentrated hydrochloric acid (37%) dropwise under stirring, react at 85°C for 2.5h, cool with dichloro The crude product was obtained by extraction with methane and precipitation, and recrystallized with butanone to obtain 15.8 g of white crystals, yield 56.6%, m.p.98-99°C. 1 HNMR (CDCl 3 , 300MHz) δ: 2.561~3.667 (s, 4H, -N-CH 2 -CH 2 -N-), 3.680~4.095(s, 5H, -CH 2 -O-CH-CH 2 -), 8.146 (s, 1H, -NH); ...
Embodiment 2
[0030] Synthesis of 1-(1,2-epoxypropyl)-N-nitroimidazolidin-2-ylamine
[0031] Add 13.0g of N-nitroimidazolidine, 13.8g of potassium carbonate and 200ml of acetone solvent into the reaction flask, heat to 80°C, and after the N-nitroimidazolidine is basically dissolved, add 10.1g of epoxy chloride dropwise Propane, after heating, continue to react for 4h. After the reaction is completed, after the reactant is cooled, filter, evaporate the filtrate to dryness, add 50ml of water (can be properly heated to make it dissolve), extract with dichloromethane, and remove the solvent to obtain the crude product. The ketone was recrystallized to obtain 11.5 g of white crystals, with a yield of 61.8%.
[0032] Biological activity evaluation
[0033] 1. Determination of toxicity to aphids
[0034] spray method
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com