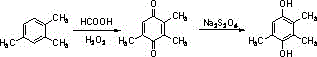
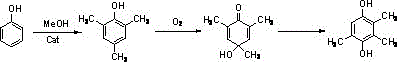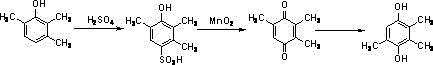A kind of synthetic method of environment-friendly 2,3,5-trimethylhydroquinone
A technology of trimethylhydroquinone and a synthesis method, applied in 2 fields, can solve problems such as high synthesis cost, difficult separation and preparation, and achieve the effects of low cost, low corrosiveness and stable reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Add 900mL of formic acid to a 3L three-necked flask equipped with a condenser tube, a calcium chloride drying tube, a constant pressure dropping funnel and a thermometer, and slowly add 1,2,4-trimethylbenzene (120g, 1moL, 1.0Eqv ), slowly raise the temperature to 50°C, as the temperature of the reaction system rises, the system gradually dissolves, after the system dissolves, slowly add 30% hydrogen peroxide (906 grams, 8moL, 8Eqv ), as the reaction occurs, the system continues to heat up, The final system temperature was kept at about 90°C for 5 hours. After the reaction was detected by TLC, the reaction system was lowered to room temperature, the system was transferred to a 10L extractor, 1600mL of toluene (800mL×2) was added to extract the product, the extracts were combined, and transferred to Add 1740 grams of 30% saturated aqueous solution of sodium dithionite to a 5L three-necked flask, and continue mechanical stirring to raise the temperature of the reaction syst...
Embodiment 2
[0044]Add 900mL of formic acid to a 3L three-necked flask equipped with a condenser tube, a calcium chloride drying tube, a constant pressure dropping funnel and a thermometer, and slowly add 1,2,4-trimethylbenzene (120g, 1moL, 1.0Eqv ), slowly raise the temperature to 50°C, as the temperature of the reaction system rises, the system gradually dissolves, after the system dissolves, slowly add 30% hydrogen peroxide (906 grams, 8moL, 8Eqv ), as the reaction occurs, the system continues to heat up, The final system temperature was kept at about 80°C for 5 hours. After the reaction was detected by TLC, the reaction system was lowered to room temperature, the system was transferred to a 10L extractor, 1600mL of toluene (800mL×2) was added to extract the product, the extracts were combined, and transferred to Add 1740 grams of 30% saturated aqueous solution of sodium dithionite to a 5L three-necked flask, and continue mechanical stirring to raise the temperature of the reaction syste...
Embodiment 3
[0046] Add 900mL of formic acid to a 3L three-necked flask equipped with a condenser tube, a calcium chloride drying tube, a constant pressure dropping funnel and a thermometer, and slowly add 1,2,4-trimethylbenzene (120g, 1moL, 1.0Eqv ), slowly raise the temperature to 50°C, as the temperature of the reaction system rises, the system gradually dissolves, after the system dissolves, slowly add 30% hydrogen peroxide (906 grams, 8moL, 8Eqv ), as the reaction occurs, the system continues to heat up, The final system temperature was kept at about 100°C for 5 hours. After the reaction was detected by TLC, the reaction system was lowered to room temperature, and the system was transferred to a 10L extractor, and 1600mL of toluene (800mL×2) was added to extract the product. The extracts were combined and transferred to Add 1740 grams of 30% saturated aqueous solution of sodium dithionite to a 5L three-necked flask, and continue mechanical stirring to raise the temperature of the react...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com