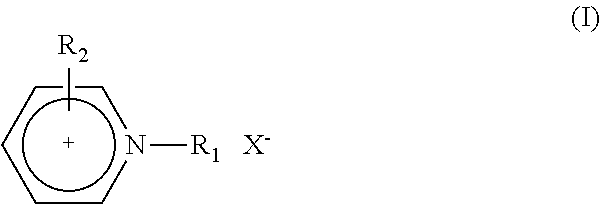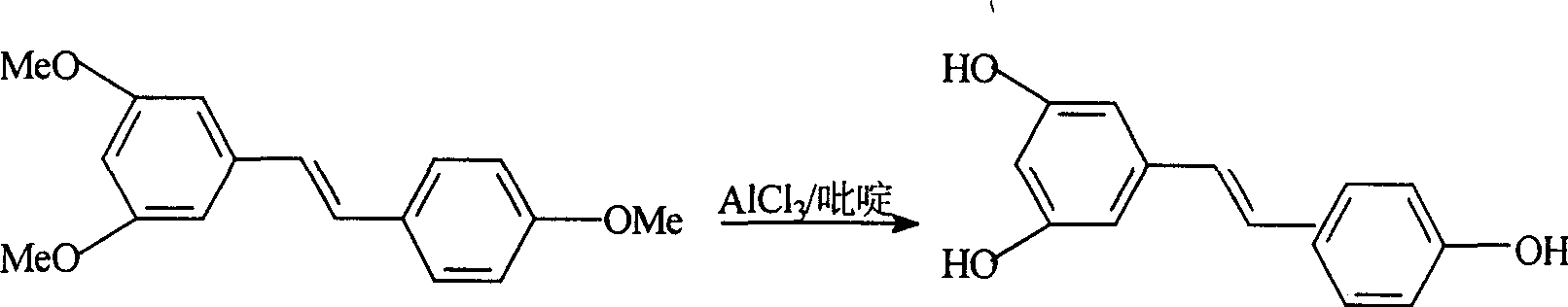Patents
Literature
508 results about "Trimethylbenzenes" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The trimethylbenzenes constitute a group of substances of aromatic hydrocarbons, which structure consists of a benzene ring with three methyl groups (–CH₃) as a substituent. Through their different arrangement, they form three structural isomers with the molecular formula C₉H₁₂. They also belong to the group of C₃-benzenes. The best-known isomer is mesitylene.
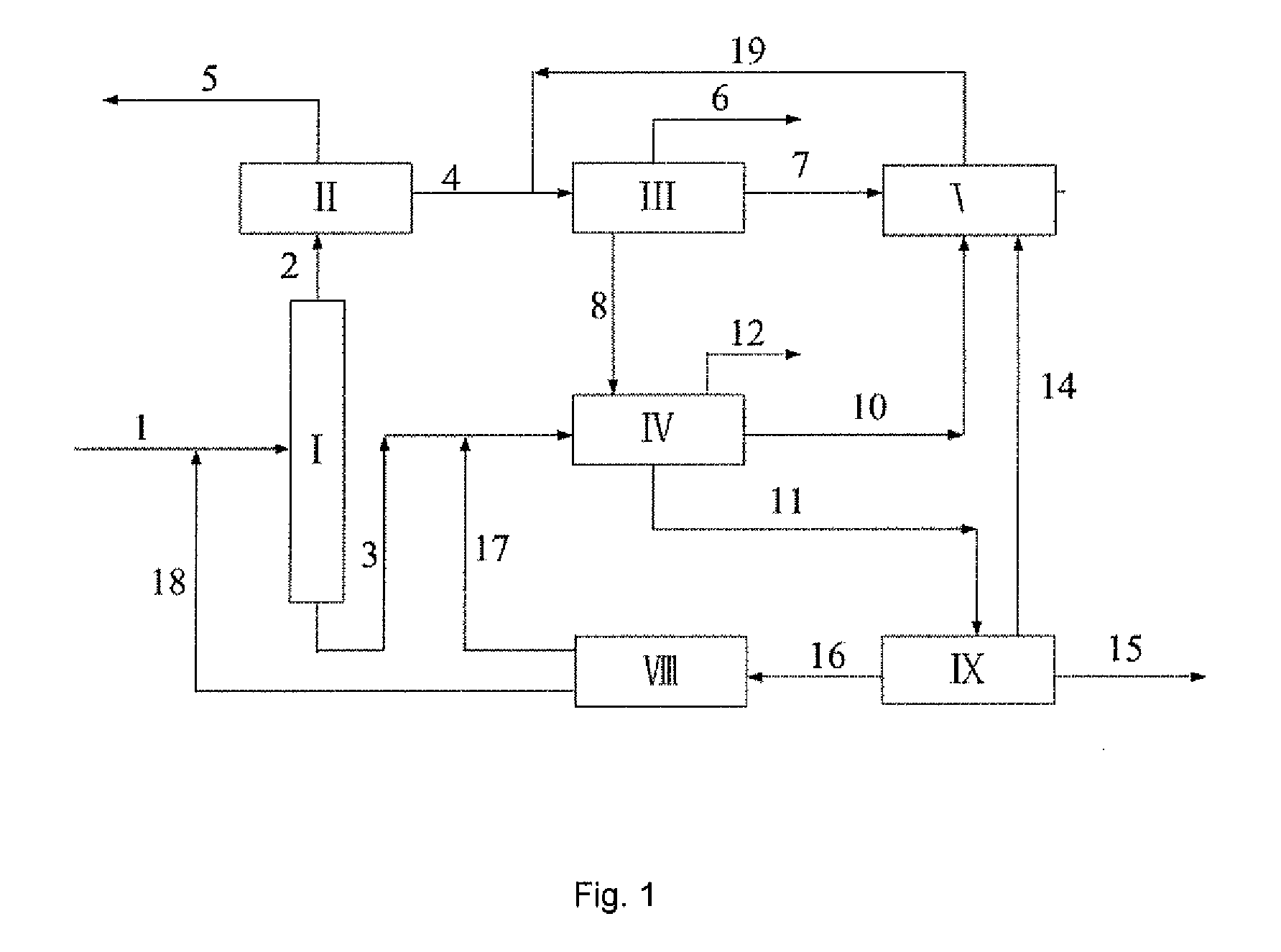
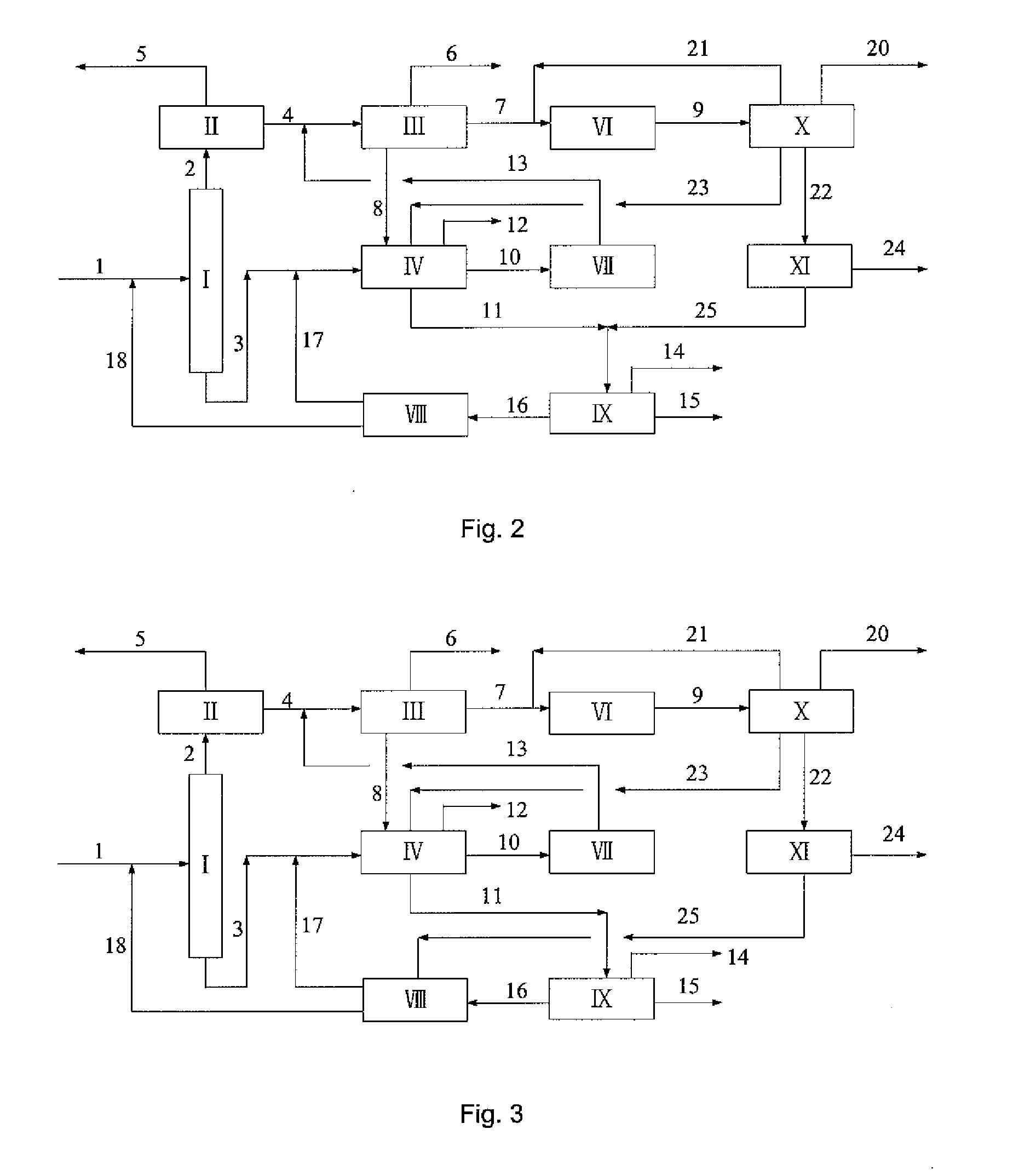
Microwave auxiliary preparation method of keto-enamine covalently linked organic framework
ActiveCN104927048ALarge specific surface areaHigh crystallinityOther chemical processesMicrowaveEnamine
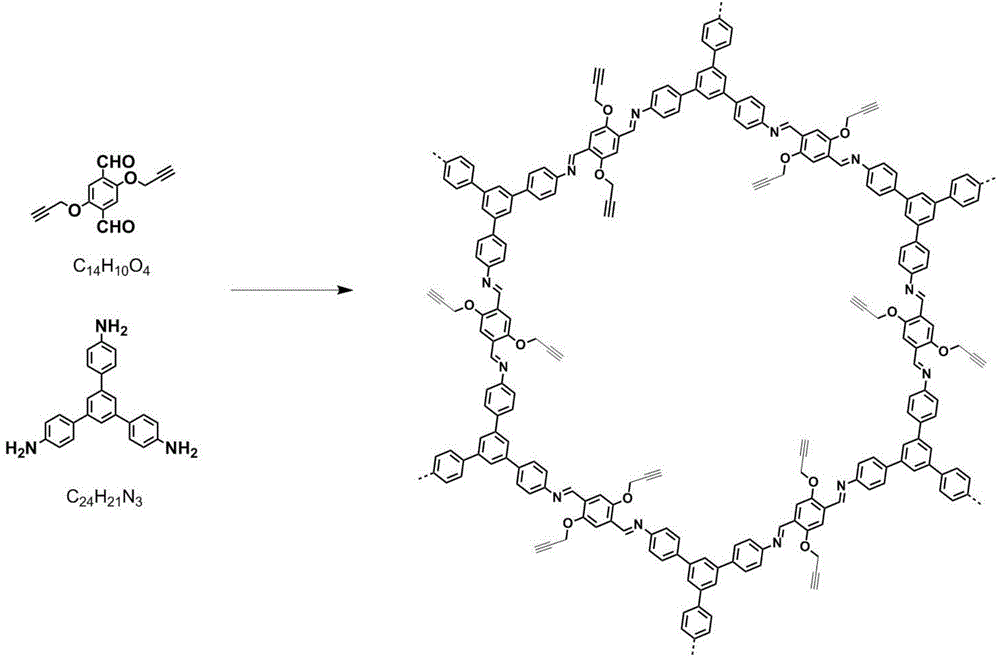
The invention discloses a microwave auxiliary preparation method of a keto-enamine covalently linked organic framework. The method comprises the following steps: charging 1 , 3 , 5-triformylphloroglucinol and p-phenylenediamine into solvent, and ultrasonically dispersing to form suspension; the solvent is mixed solvent of sym-trimethylbenzene, dioxane and acetum; freezing, by liquid nitrogen, vacuumizing and degassing the suspension for at least three times, then sealing, carrying out the thermal reaction for 1 hour under the assistance of microwaves at the temperature of 100 DEG C, and obtaining a crude product; and centrifuging the crude product, collecting insoluble matters, washing the insoluble matters sequentially by sym-trimethylbenzene and acetone to obtain the keto-enamine covalently linked organic framework, and obtaining the keto-enamine covalently linked organic framework. The method is pollution-free, the reaction condition is moderate and simple, the requirement on equipment is simple, and the method is suitable for the industrialized mass production.
Owner:芩洋科技(上海)有限公司
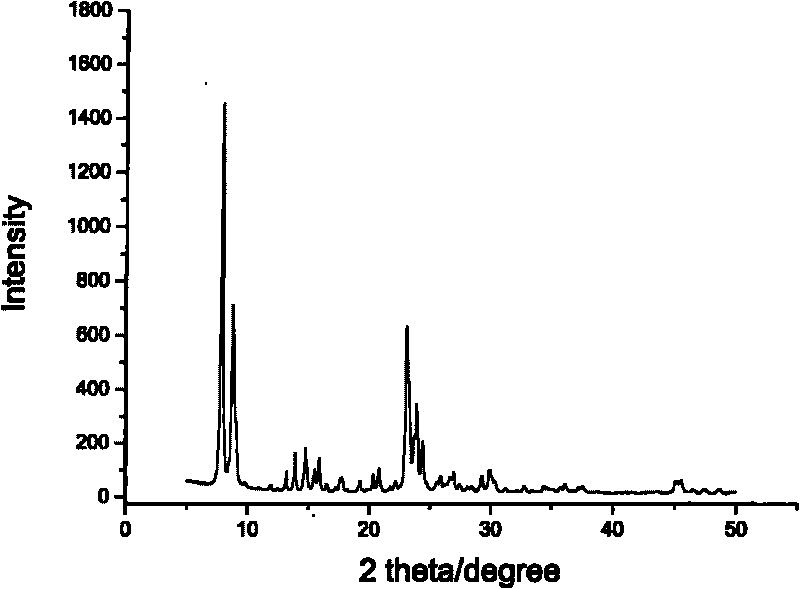
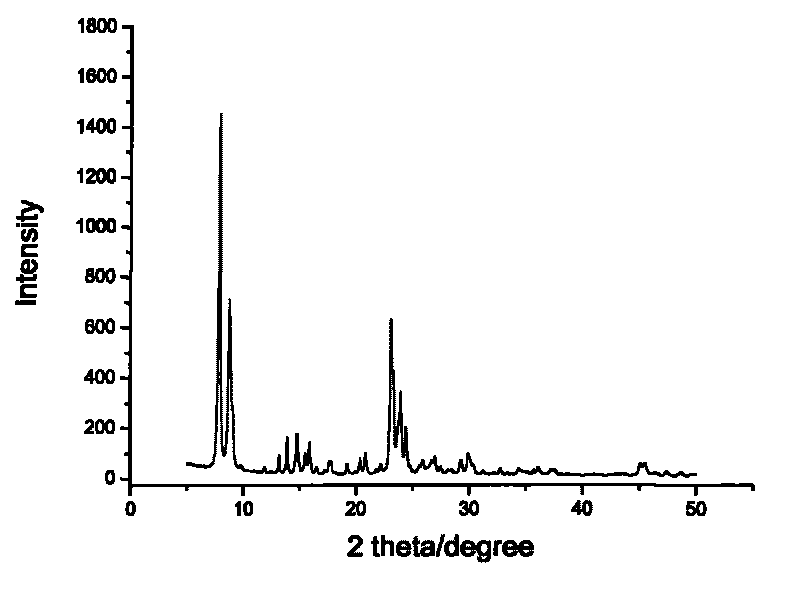
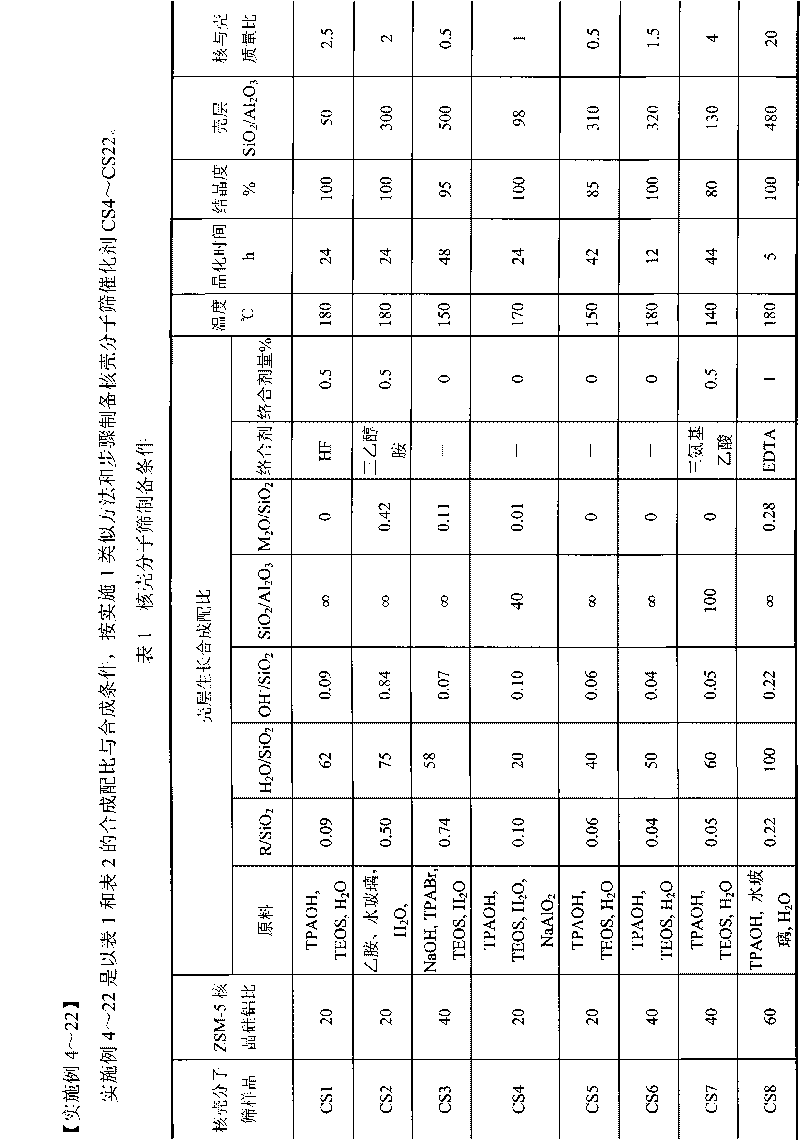
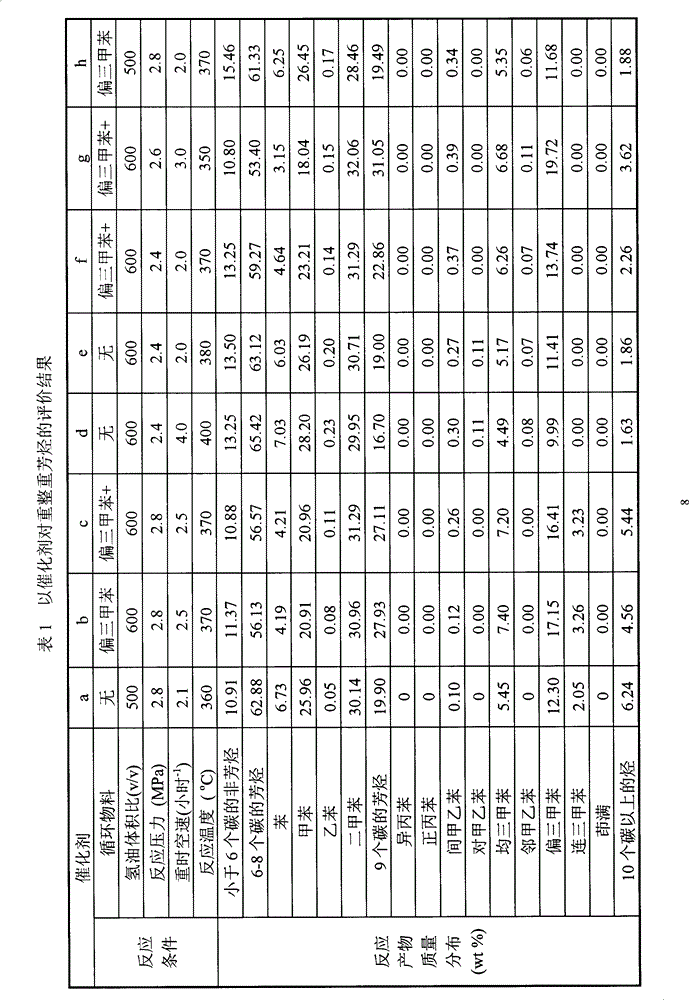
Core-shell type aromatic conversion catalyst, preparation method and application thereof
InactiveCN101722033AGuaranteed to be completely wrappedDecrease the external area char rateMolecular sieve catalystsHydrocarbon by metathesis reactionAlcoholCore shell
The invention relates to a core-shell type aromatic conversion catalyst, a preparation method and application thereof, mainly solving the problems of strong acidity of an outside surface, rapid inactivation speed and low selectivity of aromatic shape selective catalysis in previous molecular sieve based catalysts. The invention adopts the following technical scheme: the preparation method that ZSM-5 crystal grain is taken as a core and a high silicon MF1 molecular sieve shell layer is formed on the outside surface of the core crystal by secondary growth is used for synthesizing the core-shell type zeolite molecular sieve with tightly-bonded core and shell and communicated pore canals, then the catalyst is prepared by shaping and baking 10-100% of core-shell type zeolite molecular sieves and the balance binder in terms of weight percent to better solve the above problems. The invention can be used in industrial production of aromatic conversion reactions of toluene disproportionation, toluene methylation, xylene isomerisation, trimethylbenzene cracking, cumin cracking and styralyl alcohol alkylating.
Owner:CHINA PETROLEUM & CHEM CORP +1
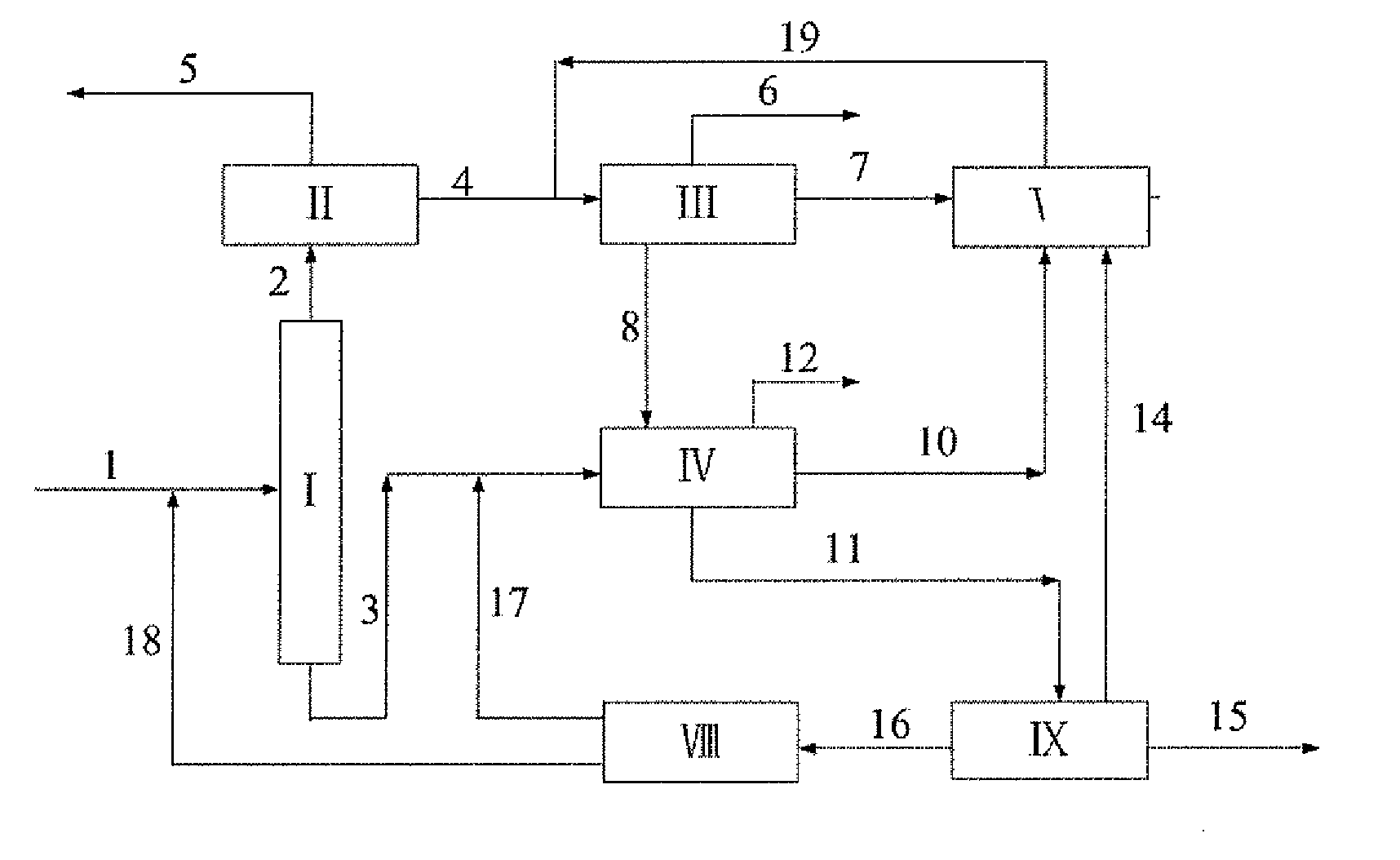
Integrated Process for the Production of P-Xylene
ActiveUS20100228066A1High energy consumptionImprove concentrationHydrocarbon by isomerisationMolecular sieve catalystsIsomerizationHydrogen
The present invention provides an integrated process for the production of p-xylene, comprising the steps of A) separating a mixed feedstock containing benzene, toluene, C8 aromatic hydrocarbons, C9 and higher aromatic hydrocarbons, and non-aromatic hydrocarbons from a reforming unit, to obtain a first benzene stream, a first toluene stream, a first C8 aromatic hydrocarbon stream, a stream of C9 and higher aromatic hydrocarbons, and a stream of non-aromatic hydrocarbons; B) feeding the stream of C9 and higher aromatic hydrocarbons from step A) to a C9 and higher aromatic hydrocarbon dealkylation unit, where dealkylation reaction occurs in the presence of hydrogen, and separating the reaction effluent to obtain a second benzene stream, a second toluene stream, and a second C8 aromatic hydrocarbon stream; C) feeding both the first toluene stream and the second toluene stream to a toluene selective disproportionation unit, where toluene selective disproportionation reaction occurs in the presence of hydrogen to produce a stream containing C8 aromatic hydrocarbons including p-xylene and benzene, which stream is separated to obtain a third C8 aromatic hydrocarbon stream, a third toluene stream, and a third benzene stream, with the third toluene stream being returned to an inlet of this unit; D) feeding both the first C8 aromatic hydrocarbon stream and the second C8 aromatic hydrocarbon stream to an adsorption separation unit, to obtain a first p-xylene product stream and a fifth C8 aromatic hydrocarbon stream, with the fifth C8 aromatic hydrocarbon stream being passed to an isomerization unit; E) feeding the third C8 aromatic hydrocarbon stream to a crystallization separation unit, to obtain a fourth C8 aromatic hydrocarbon stream and a second p-xylene product stream, with the fourth C8 aromatic hydrocarbon stream being passed to the adsorption separation unit or the isomerization unit; and F) feeding an effluent of the isomerization unit to an inlet of the adsorption separation unit.
Owner:CHINA PETROCHEMICAL CORP +1
Nematoid-killing composition containing abamectin and paecilomyces lilacinus
InactiveCN102077846AAvoid infectionIncrease parasitic rateBiocideNematocidesPaecilomyces lilacinusAbamectin
The invention relates to a nematoid-killing composition containing abamectin and paecilomyces lilacinus, which is characterized by comprising abamectin and paecilomyces lilacinus; 100g of nematoid-killing composition is prepared by the following components of 0.1-5g of abamectin, 0.1-1 billion live spores / g of paecilomyces lilacinus, 1-10g of stabilizing agent, 1-90g of solvent and 10-90g of assistant, wherein the assistant is any one or the combination of any two of attapulgite, kaoline, sepiolite, light calcium and kieselguhr and is prepared into a granular formulation or suspending agent or wettable powder; the stabilizing agent is any one or the combination of any two of castor oil, soybean oil, epoxidized soybean oil, propylene glycol, methyl oleate, ethylene glycol, butylated hydroxytoluene (BHT) and butylated hydroxyanisole (BHA); and the solvent is any one of dimethylbenzene, trimethylbenzene and dimethylformamide. The nematoid-killing composition is used for controlling the knot nematode and has better control effect on the knot nematode.
Owner:佛山市盈辉作物科学有限公司
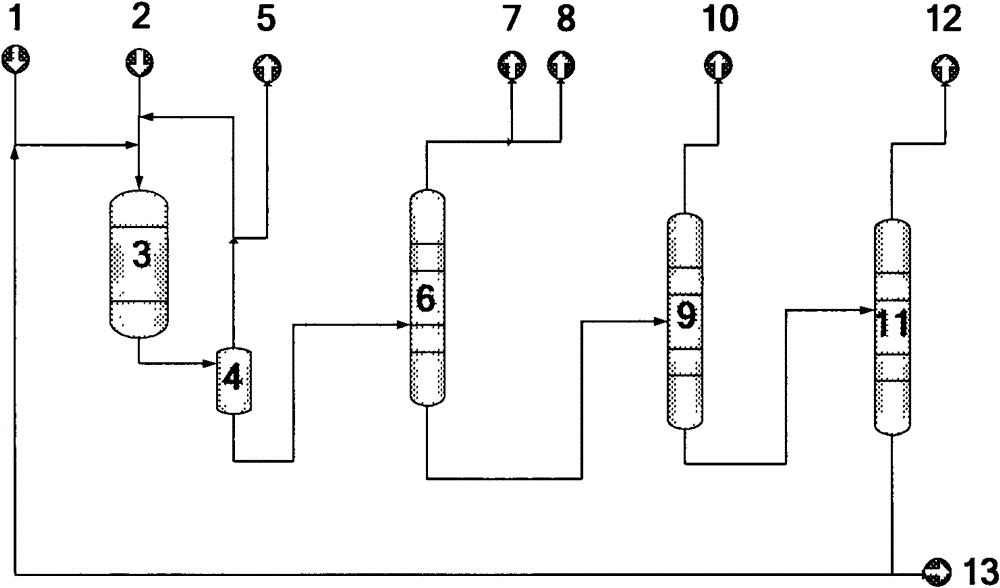
Method for separating and producing 1,3,5-trimethylbenzene through hydrocracking heavy aromatic hydrocarbons
ActiveCN102746092AEasy to removeHigh removal activityHydrocarbonsBulk chemical productionHydrogenBoiling point
The invention relates to a method for separating and producing 1,3,5-trimethylbenzene through hydrocracking heavy aromatic hydrocarbons to mainly solve technical problems of low added value of the heavy aromatic hydrocarbons, and complex flow, low product purity, low yield and the like in separation of 1,3,5-trimethylbenzene monomers from the heavy aromatic hydrocarbons. According to the method, hydrogen type binder-free ten-membered ring zeolite loading 0.005-0.5% by mass of Pt or Pd is adopted as a catalyst, hydrogen and the heavy aromatic hydrocarbons which are raw materials undergo hydrocracking treatment, and 1,2,4-trimethylbenzene and heavy aromatic hydrocarbons having boiling points greater than the boiling point of the 1,2,4-trimethylbenzene in products are returned to a reactor, so BTX aromatic hydrocarbon yield increase and 1,3,5-trimethylbenzene separation production are realized. The method well solves the problems, and can be used for the industrial production for yield increases of the BTX (benzene, toluene and xylol) aromatic hydrocarbon and the 1,3,5-trimethylbenzene.
Owner:CHINA PETROLEUM & CHEM CORP +1
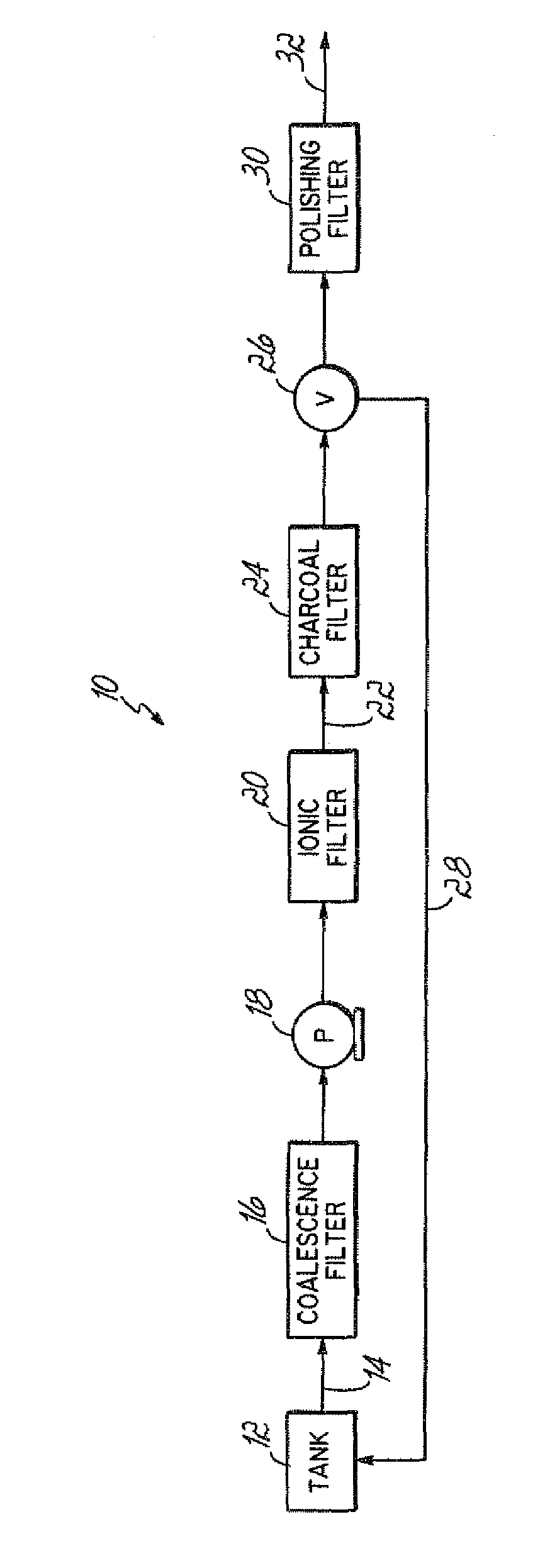
Biodiesel fuel processing
InactiveUS20070175088A1Low viscosityShorten the timeDewatering/demulsification with mechanical meansBiofuelsBiodieselNaphtha
Biodiesel is formed by blending an with a hydrocarbon fuel, preferably #2 diesel, gasoline, or kerosene. Water in the mixture, and other impurities, are removed by adding a coalescing aid such as naphtha, xylene or naphthalene and passing the mixture through a series of filters including a coalescing filter. Optional components that can be added to the fuel include cetane additives, lubricants such as trimethylbenzene and ethylene, as well as performance enhancers such as acetone.
Owner:J F BERNS
Method for preparing acyl and bisacyl phosphine oxide or acyl and bisacyl sulfur phosphines
InactiveCN101200475AReduce manufacturing stepsReduce decreaseGroup 5/15 element organic compoundsCoatingsBenzaldehydePhosphine oxide
The invention relates to a preparation method of single acyl or two acyl phosphine oxide and single acyl or two acyl sulfur phosphine compound, in particular to a preparation method of the 2,4,6-trimethylbenzene formacyl-diphenyl phosphine oxide of the photoinitiator. The addition reaction is carried out between Trimethyl benzaldehyde and diphenyl phosphorus chloride and then the mixture is oxidized by tert-butyl alcohol peroxide. The invention adopts the addition reaction of aldehyde and halogenated phosphorus and replaces the original process of acyl chloride and halogenated phosphorus, and has the advantages of greatly shortening the preparation procedures, low cost, easy operation and quick production. The compound prepared by the invention has more than 98 percent of the 2,4,6-trimethylbenzene formacyl-diphenyl phosphine oxide and can be served as the photoinitiator for solid for producing the solidification product with the performance of paint, varnish, porcelain glaze, paint, pigment or ink, and can better satisfy the increasing market demand.
Owner:TIANJIN JIURI NEW MATERIALS CO LTD
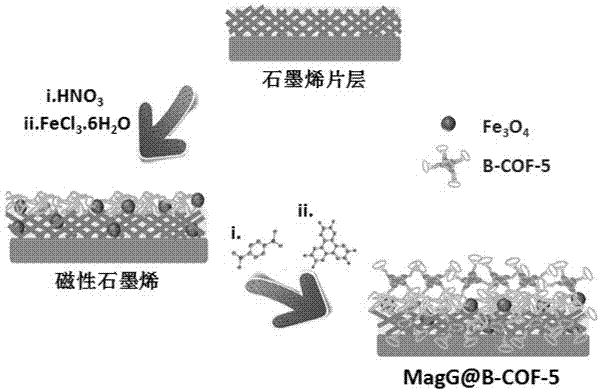
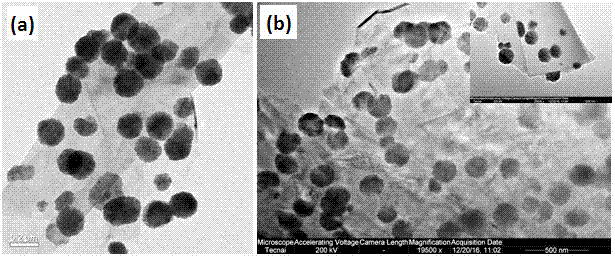
Covalent organic framework-modified graphene material and synthetic method and application thereof
InactiveCN106882797AImprove signal-to-noise ratioEfficient killingOrganic active ingredientsComponent separationBenzeneBiocompatibility Testing
The invention belongs to the technical field of novel nanometer materials, in particular to a covalent organic framework-modified graphene material, and a synthetic method and application thereof. The synthetic method comprises the following steps of firstly, a magnetic graphene material is synthesized by a one-pot method; then magnetic graphene is dispersed in a solution of 1,3,5-trimethylbenzene and 1,4-dioxane; two monomers, i.e., 1,4-phenylenebisboronic acid and 2,3,6,7,10,11-hexahydroxy triphenyl are added into the solution; and ultrasonic reaction is performed at room temperature to manufacture the covalent organic framework-modified graphene material. Experiments show that the material has a loose and porous structure, a large specific surface area and stable biocompatibility; in a concentration study of biomolecules, the composite material has the advantages of high reaction efficiency, strong specificity, high stability and the like and can be used to efficiently identify a plurality of complex mixed samples. The covalent organic framework-modified graphene material has the advantages that the cost is low, the operation is simple, and the covalent organic framework-modified graphene material is practical, efficient, high in stability and good in repeatability, and has a great application prospect.
Owner:FUDAN UNIV
Recycle of transalkylation effluent fractions enriched in trimethylbenzene
InactiveUS20120271084A1Reduce throughputLow costHydrocarbon by isomerisationDistillation purification/separationDownstream processingReaction zone
Methods are disclosed for producing C8 aromatic hydrocarbons. Representative methods comprise fractionating a transalkylation effluent, exiting a transalkylation reaction zone and comprising C8 and C9 aromatic hydrocarbons, to provide a C8 aromatic hydrocarbon-enriched fraction and a C9 aromatic hydrocarbon-enriched fraction. The methods may further comprise (i) recycling the C9 aromatic hydrocarbon-enriched fraction to the transalkylation reaction zone and / or (ii) separating, in a xylene separation zone, isomers of C8 aromatic hydrocarbons in the C8 aromatic hydrocarbon-enriched fraction, into a para-xylene-enriched extract and a para-xylene-depleted raffinate. Performance in the transalkylation reaction zone is improved and / or downstream processing requirements in an aromatics complex are mitigated.
Owner:UOP LLC
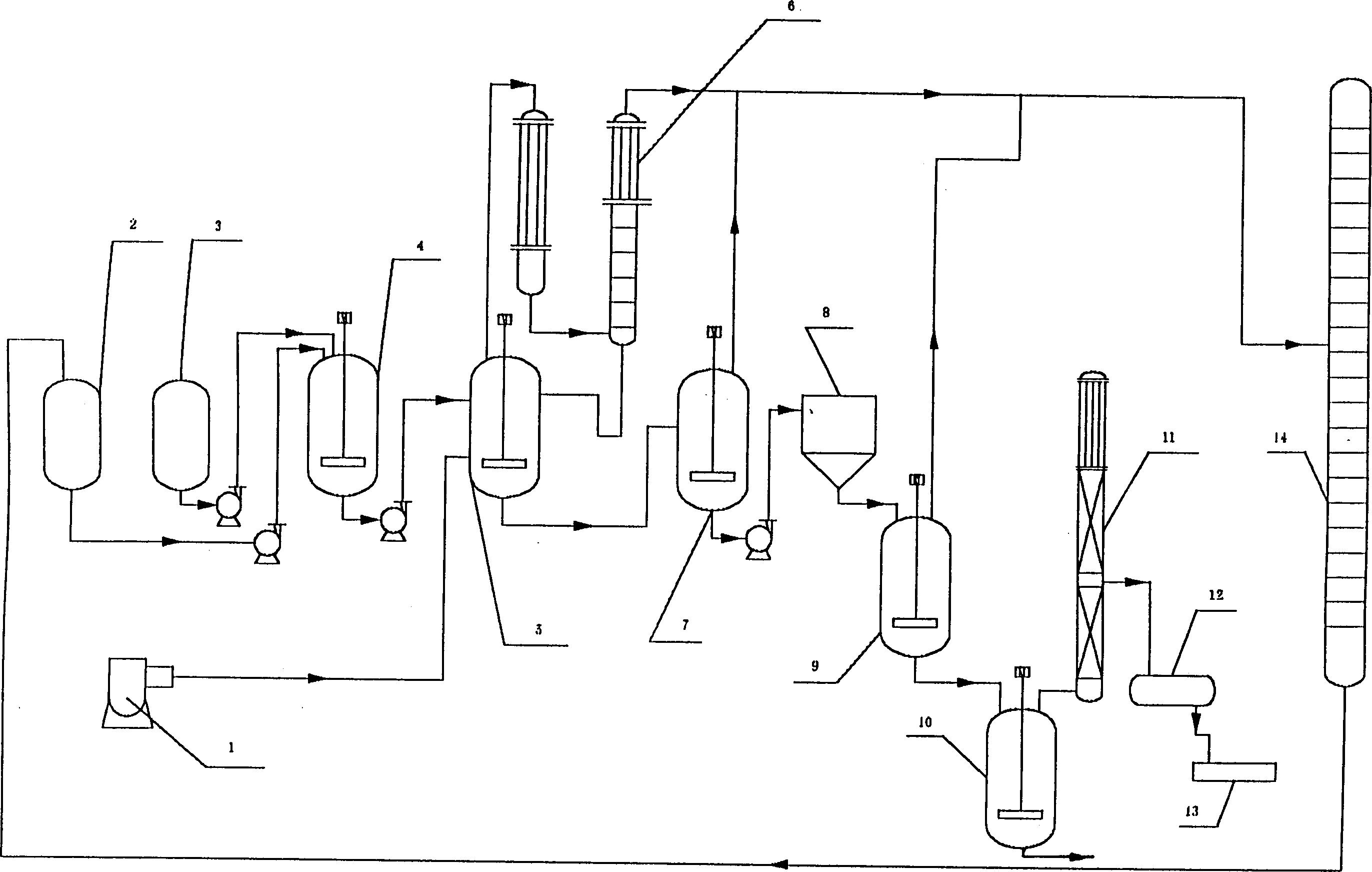
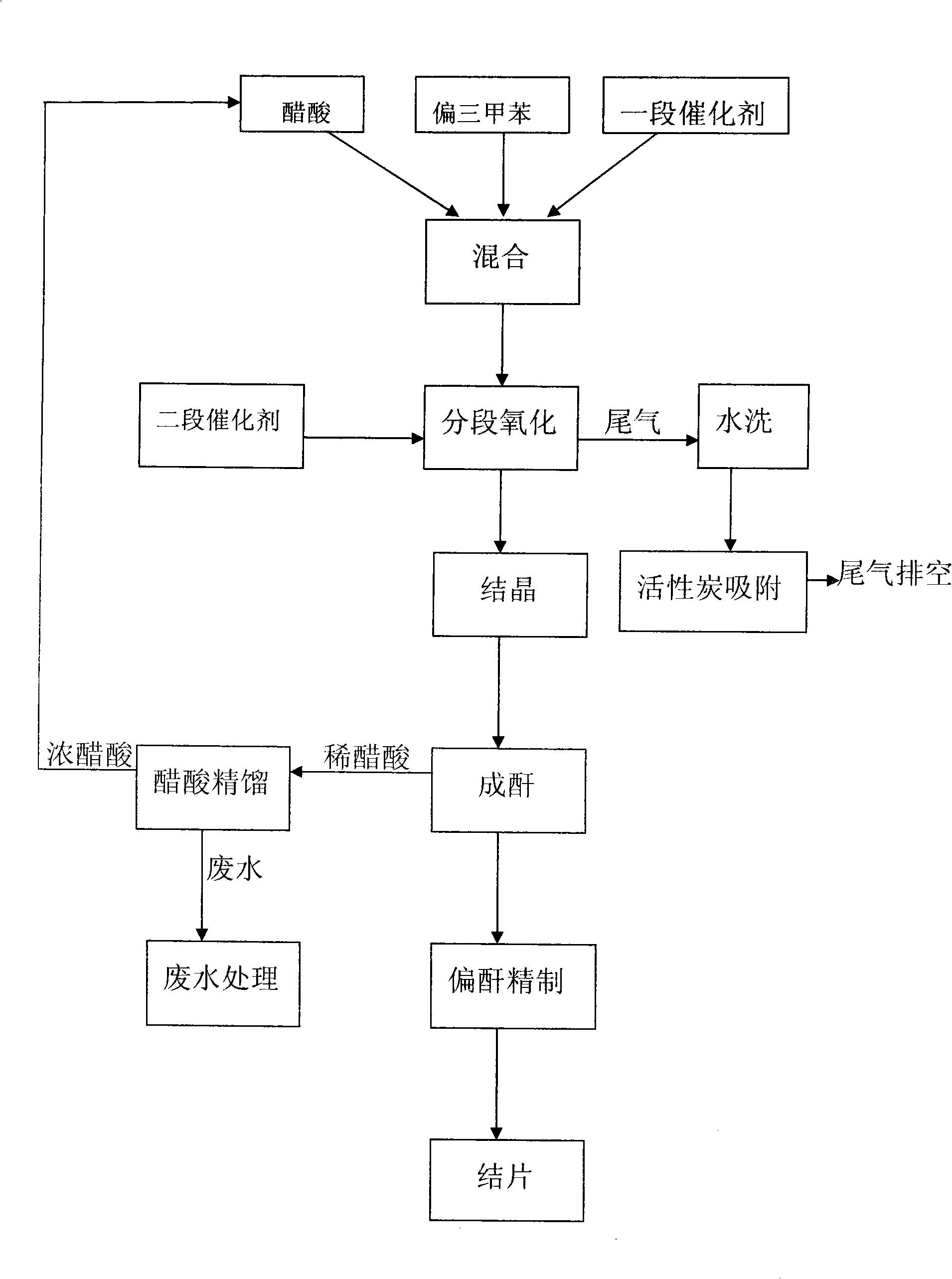
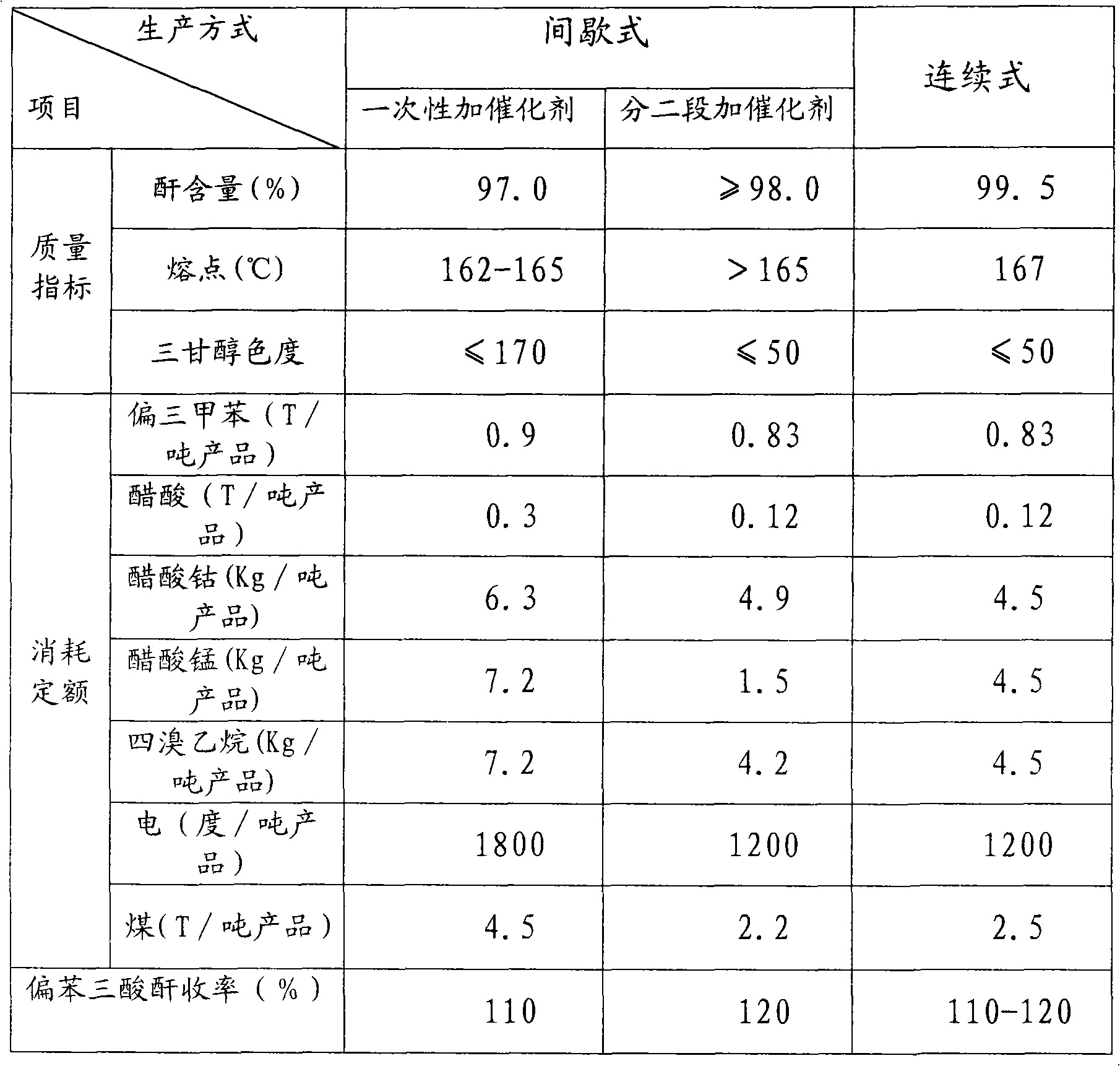
Process for production of trimellitic anhydride by continuous oxidizing process
A process for preparing trimellitic anhydride from metatritoluene, acetic acid as solvent, and catalyst (cobalt acetate, manganess acetate, tetrebromoethane, or hydrogen bromide) by continuous oxidization method includes continuous oxidizing reaction, anhydridization, refining, slicing or granulating and recovering solvent. Its advantages are high yield (up to 120%), high safety, high quality of product, and long service life of equipment.
Owner:JIANGSU ZHENGDAN CHEM IND CO LTD
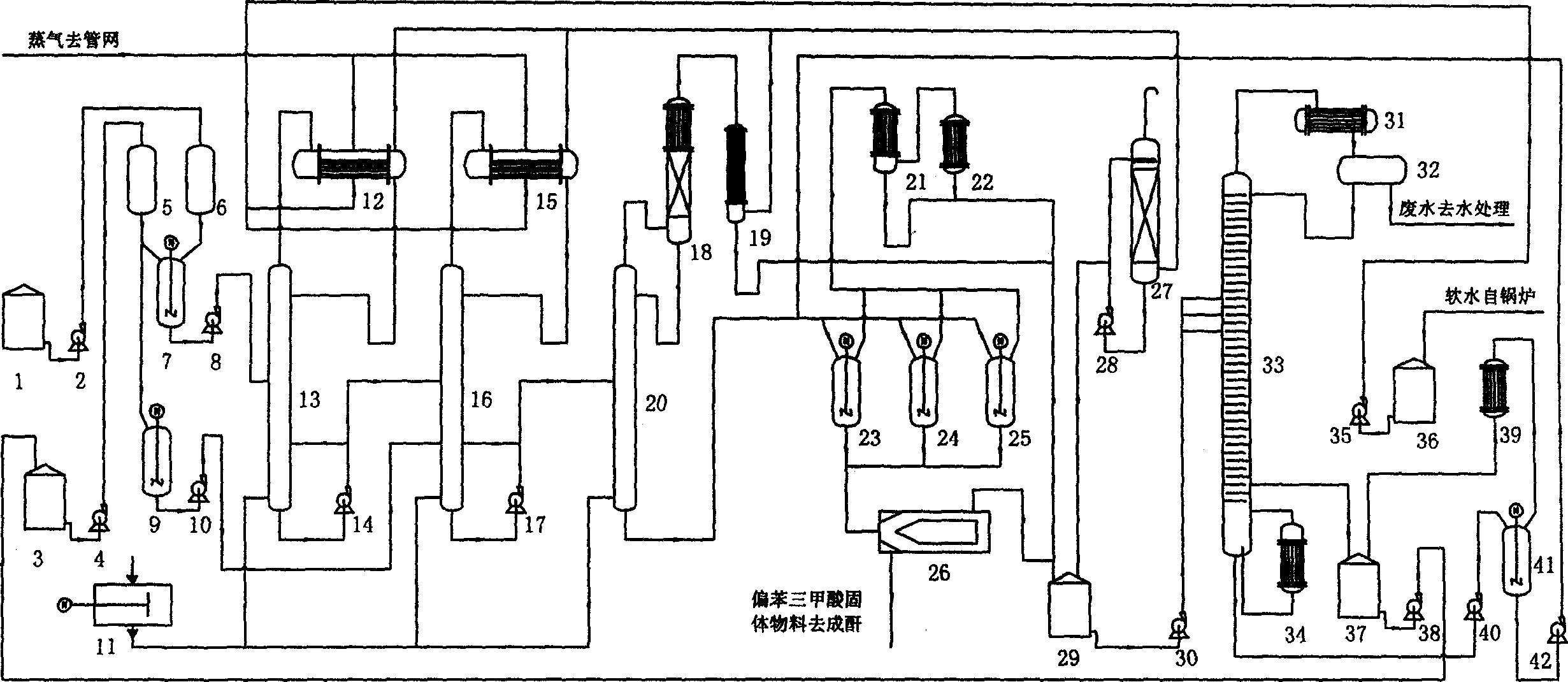
Method for producing meta benzene tricarbonic acid through sequential oxidation by using intermittent multiple cascaded Bubbling oxidation towers
ActiveCN1915960AAvoid easy-to-block problemsLess prone to damageOrganic compound preparationCarboxylic compound preparationSlurrySolvent
This invention relates to a method for preparing trimellitic acid with series intermediate bubble oxidization columns by continuous oxidation. The method comprises: (1) mixing 1,2,4-trimethylbenzene with solvent and catalyst in a kettle, heating for mixing, and sending to a series of three oxidization columns for reaction; (2) introducing the reaction products into a crystallization kettle, crystallizing to obtain mixed slurry of trimellitic acid crystal, acetic acid and water, sending to a centrifugation separator for separation, and sending the w trimellitic acid granules to the anhydridization process; (3) sending the mixture of acetic acid and water to a concentration column, and recovering acetic acid for recycling; (4) recovering trimellitic acid from the concentration column bottom product in a flash evaporation column. The series oxidization columns have such advantages as low investment, no leakage, no blockage at the inlet / outlet, continuous operation, high productivity, high yield, low raw material consumption and low energy consumption.
Owner:NANTONG BAICHUAN NEW MATERIAL CO LTD +1
Method for quickly pyrolyzing sludge to prepare bio-oil and soil improver by microwave assisted catalysis
ActiveCN105087036AIncrease contentImprove fertilitySludge treatment by pyrolysisByproduct vaporizationLiquid productSludge
The invention relates to a method for quickly pyrolyzing sludge to prepare bio-oil and a soil improver by microwave assisted catalysis. The method comprises the following steps: (1) weighing silicon carbide spherical particles and a HZSM-5 spherical catalyst in a mass ratio of 1: 1 to 1: 2, and uniformly mixing and adding the mixture into a quartz reaction kettle; (2) putting the quartz reaction kettle in a microwave splitting instrument, heating the quartz reaction kettle to 450-600 DEG C, stirring the mixture at a rate of 60-100 r / minute, gradually adding dried sludge powder into the quartz reaction kettle and condensing the generated steam to obtain a brown liquid product bio-oil, and collecting solid residues as the soil improver. The method provided by the invention is simple in process and quick to split, and the obtained split fuel is high in hydrocarbon content which can reach over 55%. The obtained split fuel comprises important chemical raw materials such as naphthalene, p-xylene, 1,3,5-trimethylbenzene, 1-methylnaphthalene, indene and the like. The obtained solid residues are very high in content of P, Ca, K and Mg and can be used as the soil improver to improve the soil fertility.
Owner:NANCHANG UNIV
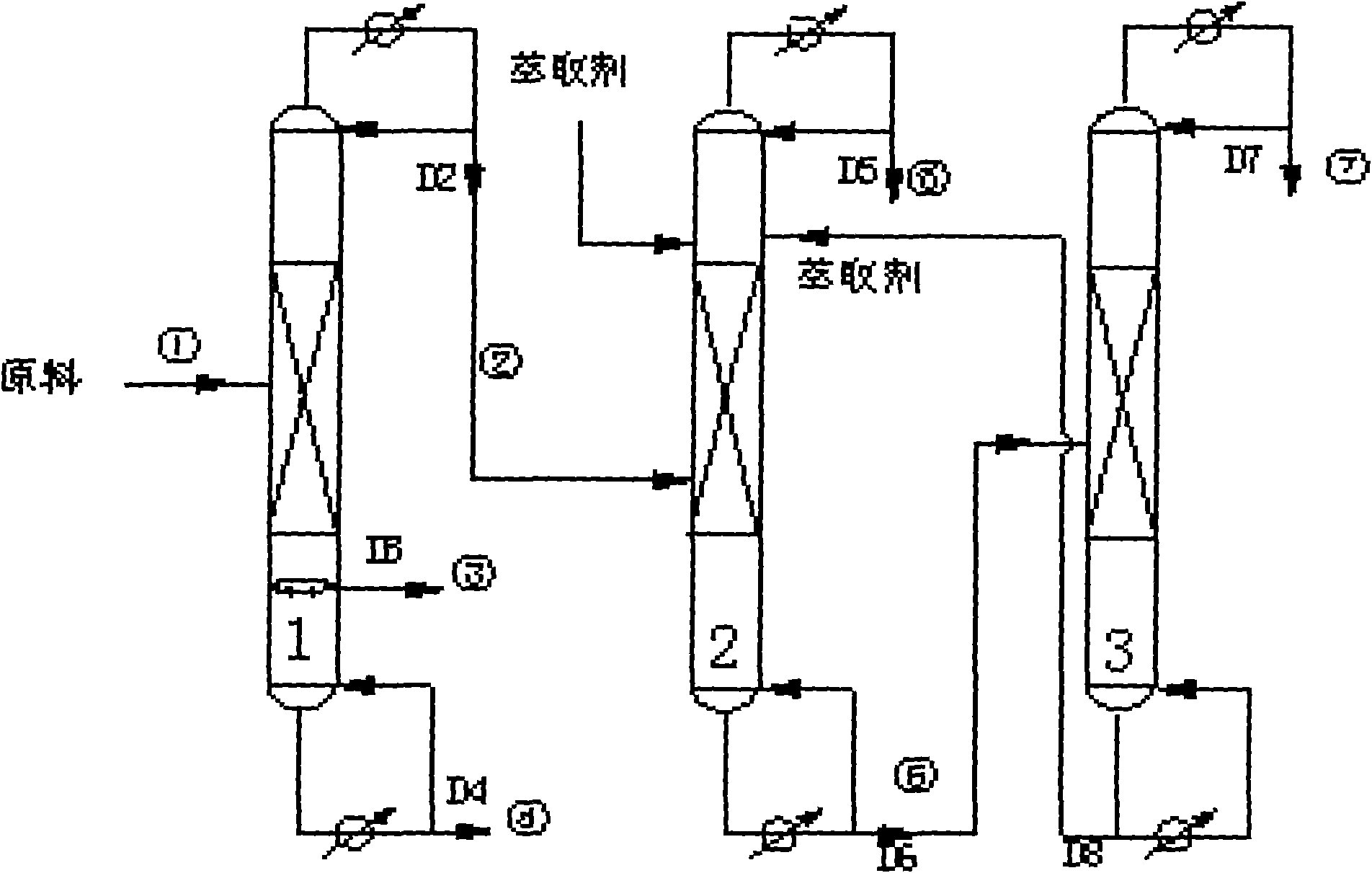
Method for extracting mesitylene fraction rich in hydrocracking C 9 by combination of continuous lateral line distillation and extractive distillation
InactiveCN101538185AIncrease contentImprove separation efficiencyDistillation purification/separationExtractive distillationTower
The invention discloses a method for extracting mesitylene fractions rich in hydrocracking C 9 by combination of continuous lateral line distillation and extractive distillation, comprising the following steps: (1) material hydrocracking C 9 aromatic is added into a continuous lateral line distillation tower, the tower bottom is heated, fractions 2 with a boiling range of 155 DEG C to 170 DEG C, fractions 3 with a boiling range of 170 DEG C to 190 DEG C and fractions 4 with a boiling range of 190 DEG C to 210 DEG C are obtained at the same time after pretreatment, content of mesitylene in the fractions 2 achieves above 90 percent, and a little mesitylene and a great deal of heavy aromatic are contained in the fractions 3 and the fractions 4; (2) the fractions 2 enter an extractive distillation tower, content of mesitylene fractions on the top 5 of the extractive distillation tower achieves above 98 percent, and fractions 6 containing a great deal of extraction solvent and heavy aromatic hydrocarbon are left at the tower bottom; (3) the fractions 6 are treated through a solvent recovery tower, heavy aromatic fractions 7 are left on the top of the solvent recovery tower, a tower reactor extracts solvent, and the solvent flows into the extractive distillation tower for recycling use. The invention only needs one distillation tower, obviously improves the efficiency, and lowers the energy consumption and the cost.
Owner:SINOPEC YANGZI PETROCHEM
Hydroxy acrylic resin for ultra-fast dry automobile varnish and preparation method thereof
InactiveCN102153691AHas ultra-fast drying and curing propertiesImprove the level ofCoatingsPolymer scienceAcrylic resin
The invention discloses a preparation method of hydroxy acrylic resin for ultra-fast dry automobile varnish. The preparation method comprises the following steps of: 1) putting a material A into a reaction kettle, and heating the material A to the temperature of between 140 and 160 DEG C; 2) dripping a material B for 3 to 4 hours, and controlling the temperature in the reaction kettle at 140 to 160 DEG C; 3) after the dripping is finished, preserving the heat for 3.5 to 4.5 hours at the temperature of between 140 and 160 DEG C; 4) adding a material C, and performing heat preservation reactionfor 3.5 to 4.5 hours; and 5) cooling the reaction product to be less than 80 DEG C, and filtering the reaction product, wherein the material A comprises 10 to 15 weight parts of dimethyl benzene and 28 to 32 weight parts of trimethyl benzene; the material B comprises 20 to 25 weight parts of styrene or alpha-styrene, 12 to 15 weight parts of sec-butyl methacrylate or tert-butyl methacrylate, 10 to 12 weight parts of propyl methacrylate or ethyl methacrylate or ethyl acrylate, 2 to 4 weight parts of methyl acrylic acid, and 0.3 to 1.0 weight part of maleic anhydride or fumaric acid; and the material C is 6 to 9 weight parts of initiator. The hydroxy acrylic resin is applied to the varnish, and has ultra-fast dry curing property, good construction performance and varnish film appearance.
Owner:同宇新材料(广东)股份有限公司
Polymers with low gel content and enhanced gas-fading
ActiveUS20100197837A1Effective amount of stabilizingImpression capsOther chemical processesLinear low-density polyethyleneEthylene Homopolymers
A polymer stabilizing composition comprising a sterically hindered phenol and a phosphite that provides low gel content and enhanced resistance to gas-fading. The stabilizer composition is particular useful for stabilizing polyethylene homopolymers and copolymers, such as linear low density polyethylenes produced from metallocene catalyst. The sterically hindered phenol is selected from the group consisting of 1,3,5-tris-(4-tert-butyl-3-hydroxy-2,6-dimethylbenzyl)isocyanurate, 1,3,5-tris-(3,5-dicyclohexyl-4-hydroxybenzyl)isocyanurate, 1,3,5-tris-(3,5-di-tert-butyl-4-hydroxybenzyl)isocyanurate, 1,3,5-tris(4-t-butyl-3-hydroxy-2,6-dimethylbenzyl)-1,3,5-Triazine-2,4,6-(1H,3H,5H)-trione, and 1,3,5-tris-(3,5-di-tert-butyl-4-hydroxybenzyl)-2,4,6-trimethylbenzene. The phosphite preferably is a liquid phosphite composition comprising two or more alkylated aryl phosphites.
Owner:SI GRP USA USAA LLC
Method for increase production of BTX (benzene, toluene and xylol) aromatic hydrocarbons and trimethylbenzene through hydrocracking heavy aromatic hydrocarbons
ActiveCN102746093AEasy to removeHigh removal activityHydrocarbonsBulk chemical productionBenzeneHydrogen
The invention relates to a method for the increase production of BTX aromatic hydrocarbons and trimethylbenzene through hydrocracking heavy aromatic hydrocarbons to mainly solve technical problems of low heavy aromatic hydrocarbon added value and high cost for separating and producing trimethylbenzene monomers in the heavy aromatic hydrocarbons containing methylethylbenzene, propylbenzene and butylbenzene. The method comprises a step that the heavy aromatic hydrocarbons are subjected to a hydrocracking reaction through adopting Pt / Pd-loaded hydrogen type binder-free ten-membered ring zeolite as a catalyst under reaction conditions comprising that the reaction temperature is 320-450DEG C, the reaction pressure is 2.0-4.0Mpa, the hydrocarbons raw material weight hourly space velocity is 1.0-4.0h<-1> and the hydrogen / hydrocarbon raw material ratio by mole is 3-10:1. The method well solves the problems through above technical scheme, and can be applied to the industrial production of the BTX aromatic hydrocarbons and the trimethylbenzene.
Owner:CHINA PETROLEUM & CHEM CORP +1
Catalyst for yield increases of BTX (benzene, toluene and xylol) aromatic hydrocarbons and trimethylbenzene through hydrocracking heavy aromatic hydrocarbons
The invention relates to a catalyst for the yield increases of BTX aromatic hydrocarbons and trimethylbenzene through hydrocracking heavy aromatic hydrocarbons to mainly solve technical problems of insufficient capability of present hydrotreatment catalysts for removing C9 aromatic hydrocarbons except the trimethylbenzene from the heavy aromatic hydrocarbons with low added values, and long flow, difficult separation and high cost in rectification separation of trimethylbenzene monomers from products. According to the invention, hydrogen type binder-free ten-membered ring zeolite loading 0.005-0.5 parts by mass of Pt or Pd is adopted as the catalyst, so the catalyst well solves the problems, and can be used for the industrial production for the yield increases of the BTX aromatic hydrocarbons and the trimethylbenzene with a high added value through hydrocracking the heavy aromatic hydrocarbons.
Owner:CHINA PETROLEUM & CHEM CORP +1
Determination method of harmful organic substance residue in toy sample
InactiveCN102608225AShorten the timeImprove efficiencyComponent separationCyclohexanoneMethoxyacetic acid
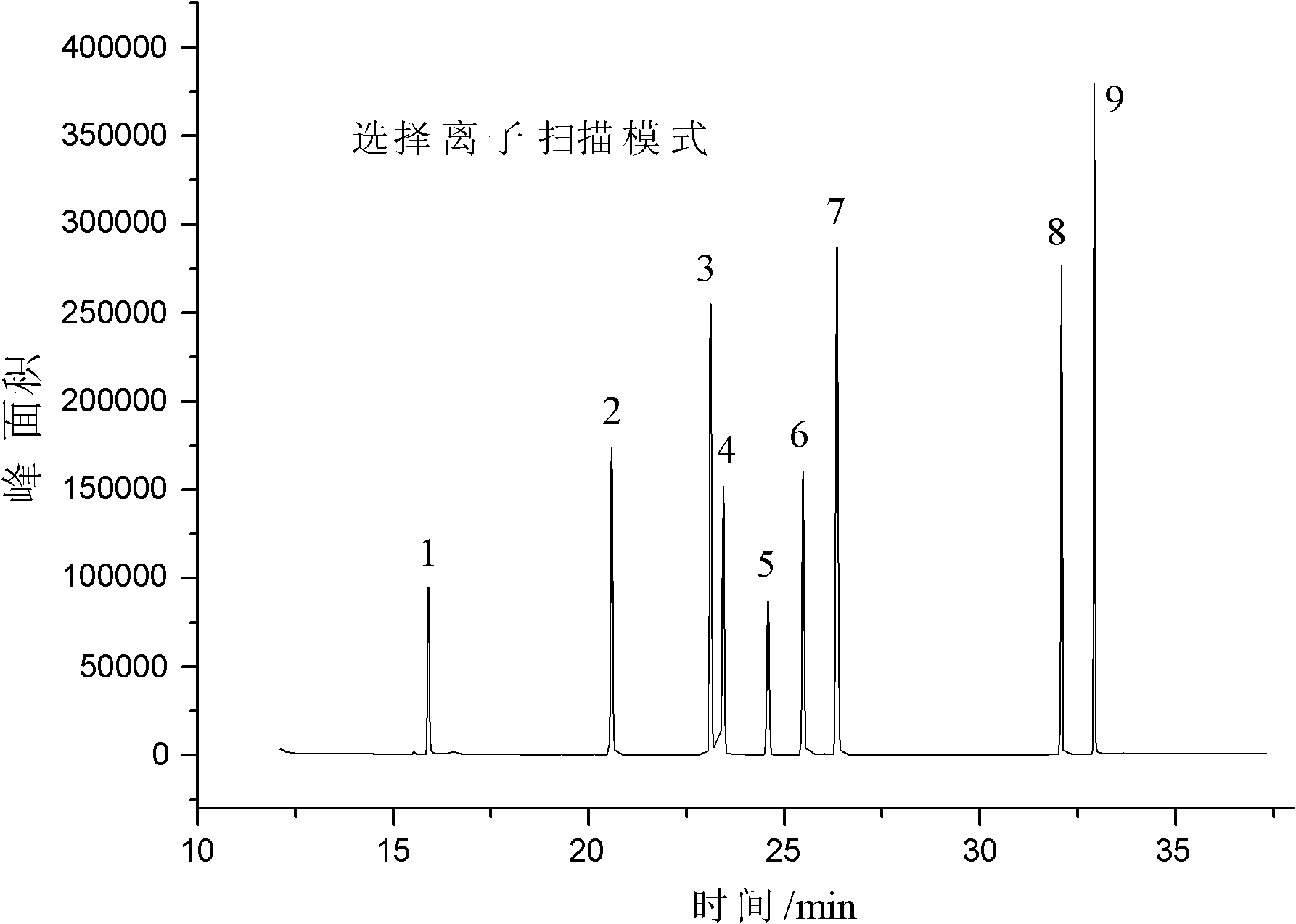
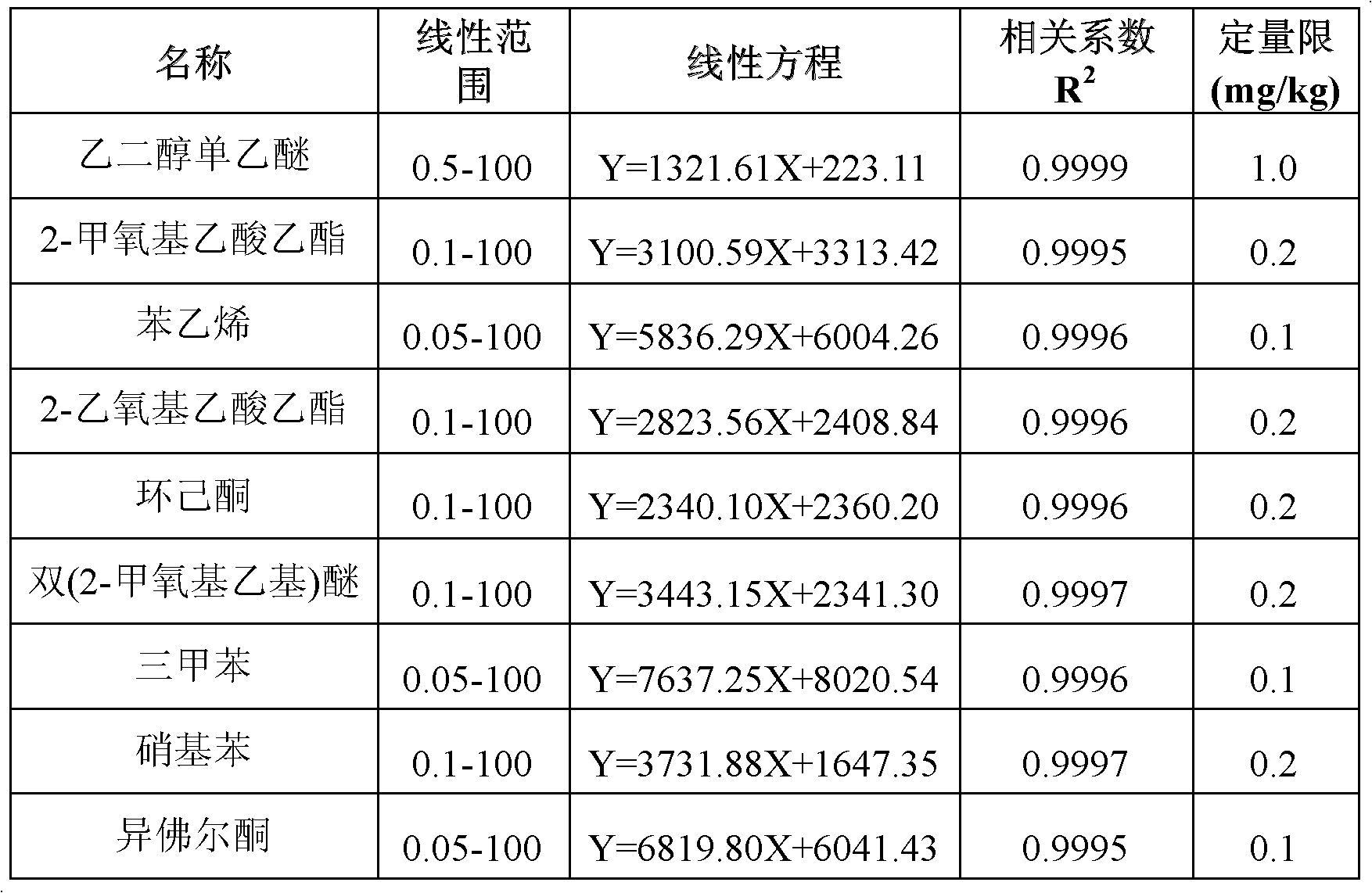
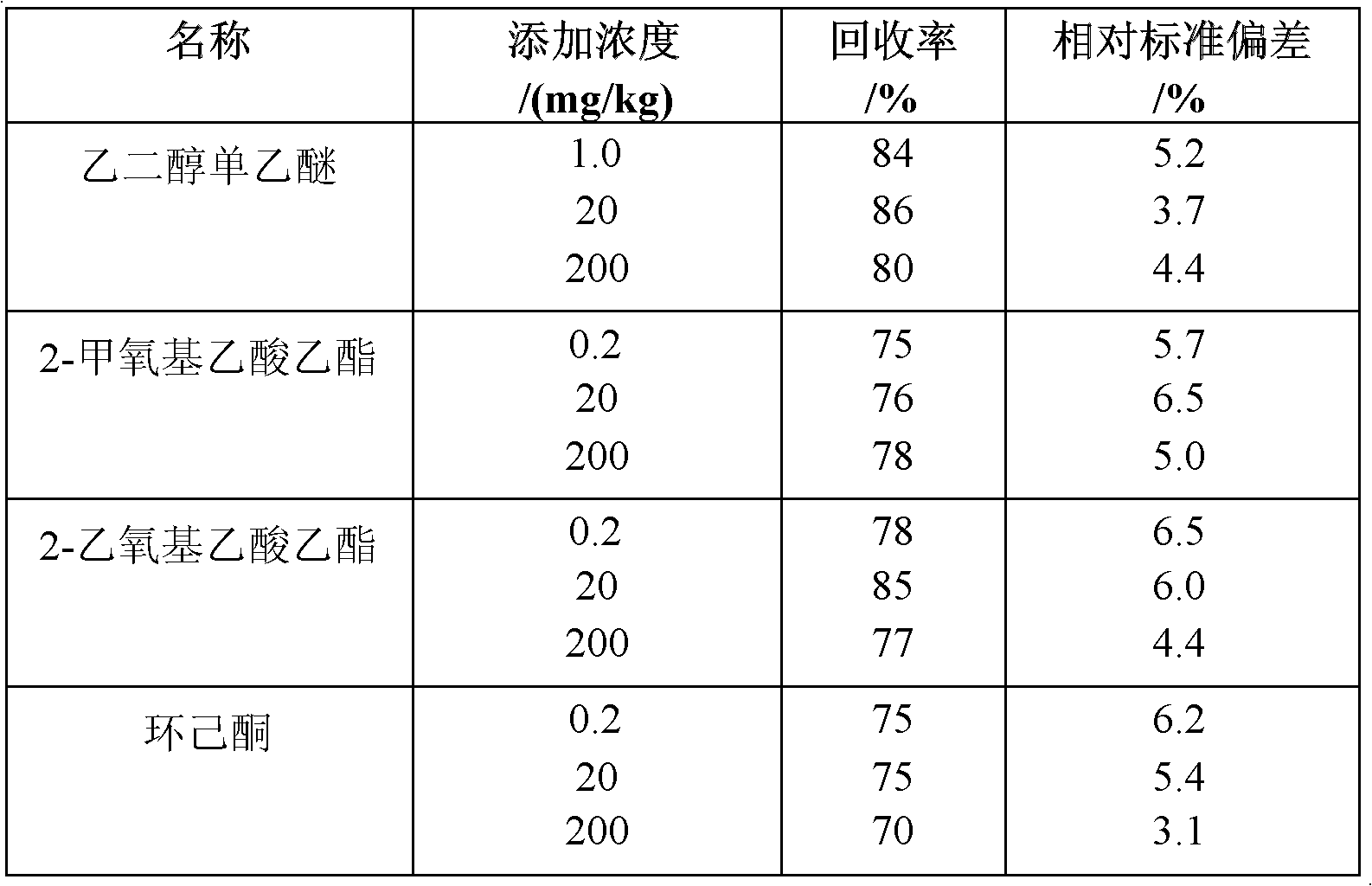
The invention adopts gas chromatography-mass spectrometry (GC-MS) to originally simultaneously determine residual contents of 9 kinds of organic substances in toy, including ethylene glycol monoethyl ether (EGEE), styrene, trimethylbenzene, isophorone, 2-methoxyethyl acetate, 2-ethoxyethyl acetate, cyclohexanone, bis(2-methoxyethyl) ether, and nitrobenzene. Common detection device is adopted in the method. The method provided by the invention has the advantages of easy popularization, simultaneous detection to save time, improved efficiency, low cost, high detection rate and sensitivity, and high application value in toy production and quality detection.
Owner:CHINESE ACAD OF INSPECTION & QUARANTINE
Gloss oil for IMD (In-Mold Decoration) printing ink, IMD printing ink, IMD film and preparation method of gloss oil
The invention relates to gloss oil for IMD (In-Mold Decoration) printing ink, IMD printing ink, an IMD film and a preparation method of the gloss oil. The gloss oil for IMD printing ink consists of the following components by weight percent: 25-30% of cyclohexanone, 15-20% of isophorone, 15-20% of trimethylbenzene, 15-20% of Shenzhen PuRuiSen polyester resin ES-300 and 25-30% of Shenzhen PuRuiSen polyester resin ES-410. The preparation method of the gloss oil comprises the following steps: adding the cyclohexanone, isophorone and trimethylbenzene in a blender, turning on the blender, adding the polyester resin ES-300 and the polyester resin ES-410 during stirring, and stirring uniformly. Compared with the prior art, the invention has the advantages that the IMD film printed with the IMD printing ink prepared from the gloss oil provided by the invention does not deform during high-temperature injection, and the adhesion of the printing ink is good; and the curing agent prepared from 70% of 1,6-diisocyanatohexane and 30% of toluene diisocyanate can ensure the yellowing resistance and enhance the hardness of the printing ink at the same time.
Owner:中山市中益油墨涂料有限公司
Method for preparing mesoporous hydroxyapatite nanometer particle with high specific surface area by virtue of template method
ActiveCN104192817ALarge specific surface areaIncrease loadMaterial nanotechnologyPhosphorus compoundsWater bathsApatite
The invention discloses a method for preparing a mesoporous hydroxyapatite nanometer particle with high specific surface area by virtue of a template method. The method comprises the following steps: firstly, mixing calcium pantothenate, F123 and a certain amount of sym-trimethylbenzene to prepare an emulsion and then dropwise adding a phosphate solution with a certain pH value into the emulsion, heating in a water bath, carrying out reflux reaction and filtering to obtain a precipitate and finally calcining the precipitate in a muffle furnace to remove a template to obtain the hydroxyapatite nanometer particle with a mesoporous structure. The mesoporous hydroxyapatite prepared by the method disclosed by the invention has the characteristics of high specific surface area, large drug loading amount and uniform pore size distribution and is distributed in a spherical nanometer state (the size is less than 100nm). The mesoporous hydroxyapatite serving as a drug carrier can well penetrate through blood vessels and cell walls to reach diseased cells. The entire preparation process is simple and the mesoporous hydroxyapatite can be produced in a large scale.
Owner:HARBIN INST OF TECH
Separation of mesitylene from 1,2,4-trimetyhlbenzene by azeotropic distillation
InactiveUS6136155AUsefulness and utilityEasy to separateDistillation purification/separationHydrocarbonsAmyl alcoholBoiling point
Mesitylene is difficult to separate from 1,2,4-Trimethylbenzene because of the proximity of their boiling points. They are readily separated by azeotropic distillation. Effective agents are isopropyl acetate, 2-pentanol and acetonitrile.
Owner:BERG LLOYD
Method for producing trimellitic anhydride with pseudocumene liquid phase air segmenting hydrocarbonylation
The invention relates to a method for producing trimellitic anhydride by using a pseudocumene liquidoid air subsection oxidation method. Pseudocumene is used as a raw material, acetic acid is used as a solvent, and cobalt acetate, manganese acetate, and tetrabromoethane are used as catalysts; the solvent weight ratio of pseudocumene to acetic acid is between 1 to 2.5 and 1 to 10; and the weight ratio of the total catalysts to the pseudocumene is as follows: the ratio of the pseudocumene to the cobalt acetate to the manganese acetate to the tetrabromoethane is 1 to 0.005-0.05 to 0.005-0.05 to 0.005-0.05. The method comprises the following: a step of mixture, in which the pseudocumene and the acetic acid are weighed out according to the proportion, are added with the cobalt acetate and the tetrabromoethane which account for 40 to 60 percent of the total weight at a temperature between 60 and 100 DEG C; a step of subsection oxidation, in which the mixed materials enter the first reaction period which lasts for 30 to 50 minutes from the beginning of the oxidation, at a temperature between 140 and 180 DEG C and at a pressure of between 0.4 and 1.0 Mpa, then enter the second reaction period at a temperature of 180 and 300 DEG C and at a pressure of between 1.0 and 3.0 Mpa, and finally are added with the residual catalysts; a step of anhydride formation; and a step of fine purification. The method effectively solves the problem of self-inhibition in oxidation reaction.
Owner:安徽泰达新材料股份有限公司
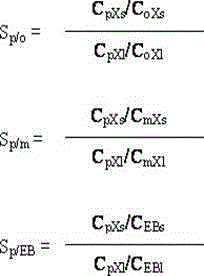
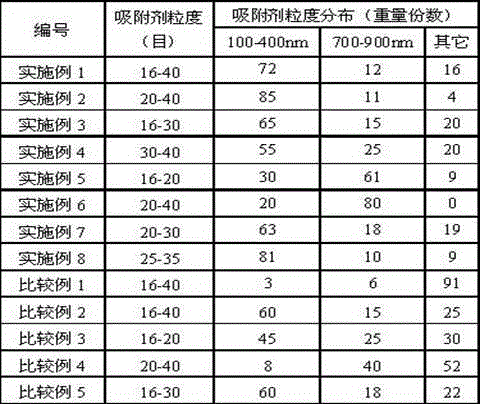
High-silicon MFI zeolite adsorbent without adhesive and preparation method thereof
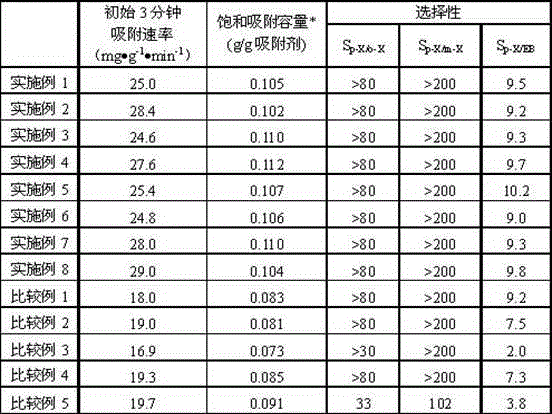
The invention relates to a high-silicon MFI zeolite adsorbent without adhesive and a preparation method thereof, which mainly aim at solving the problems of small adsorption capacity of an adsorbent for PX in mixed xylene C8, as well as low adsorption rate and poor separation effect in the prior art. The high-silicon zeolite adsorbent without adhesive is prepared through the steps of adding high-silicon MFI powder in specific grain size distribution into a silicon adhesive for forming and performing crystal transformation, wherein the grain size of the adsorbent is 16-40 meshes; at room temperature, when a solid-liquid ratio is 3 to 5, the adsorption capacity of per gram of adsorbent for p-xylene in a 1,3,5-trimethylbenzene solution containing 10% of p-xylene based on weight percent is greater than 0.102g, the selectivity coefficient of p-xylene for ethylbenzene, o-xylene and m-xylene is higher than 9.0, and within initial three minutes, the adsorption rate of the per gram of adsorbent for p-xylene is greater than 22mg / minute. By virtue of the technical scheme, the technical problems are well solved and the high-silicon MFI zeolite adsorbent without adhesive can be used for separation of p-xylene in C8 arene.
Owner:CHINA PETROLEUM & CHEM CORP +1
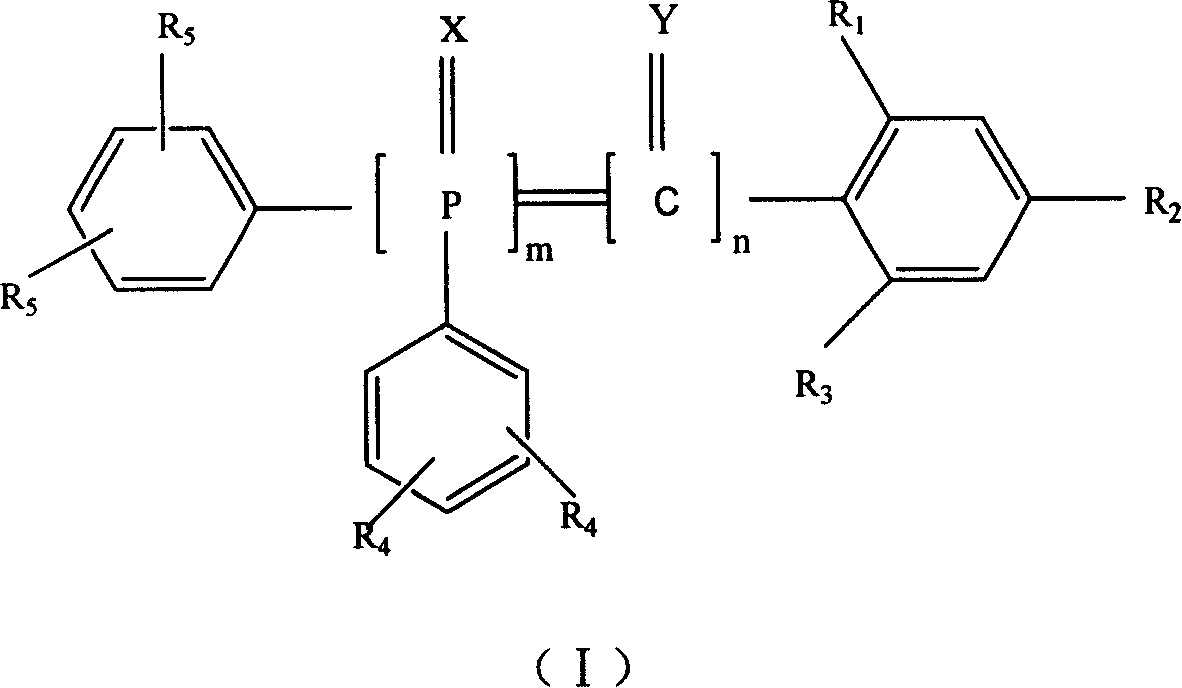
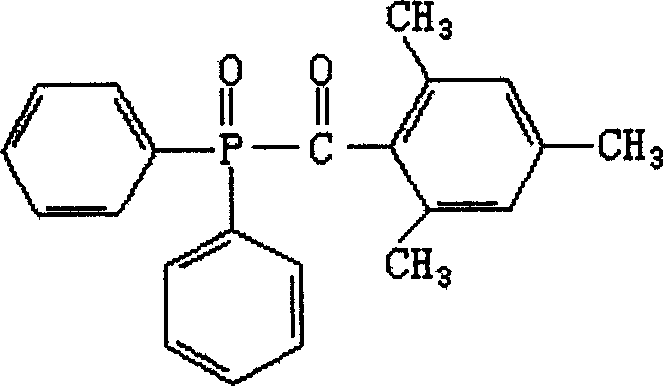
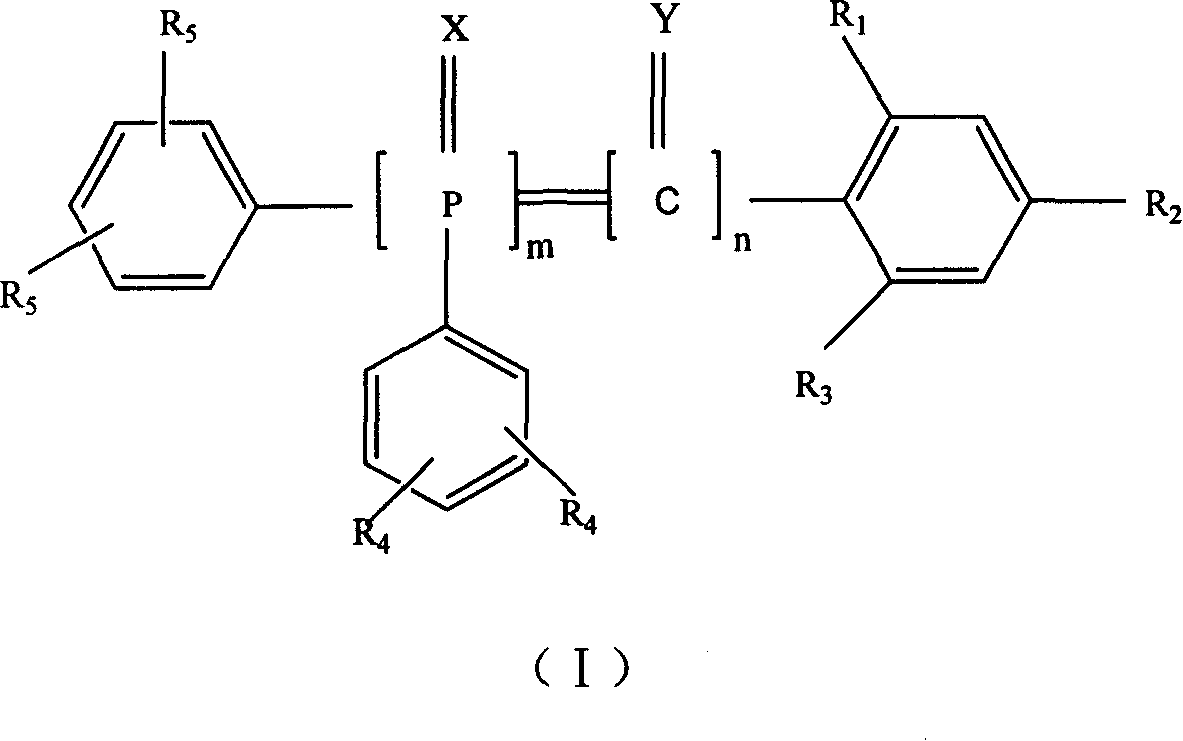
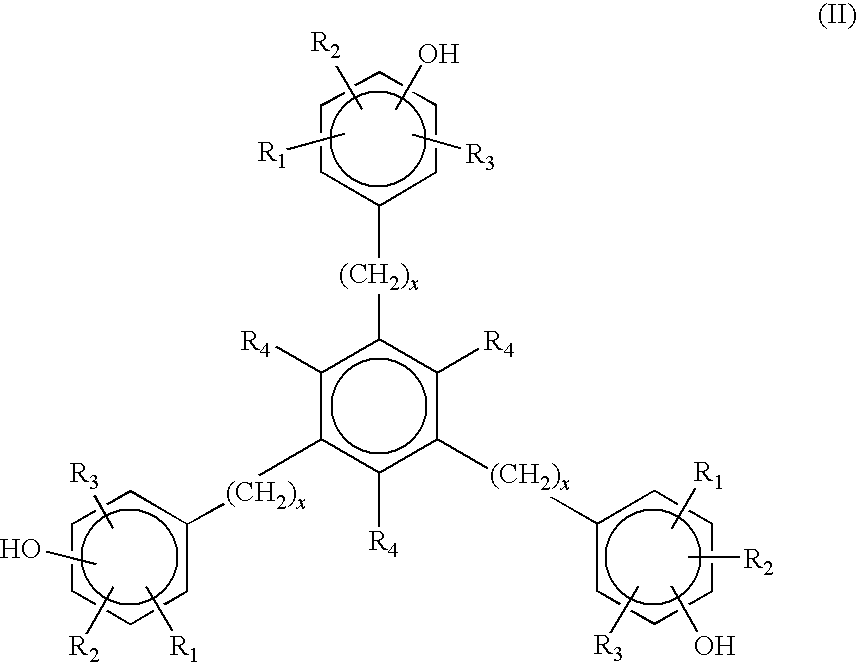
6-trimethylphenyl-6H-6-boroheterobenzo[cd]pyrene derivatives and application thereof
ActiveCN103665014AImprove film formationGood thermal stabilitySolid-state devicesSemiconductor/solid-state device manufacturingOrganic electroluminescenceChemistry
The invention relates to compounds disclosed a Formula (1), wherein n is 1 or 2; R1-R5 are identical or different, and are respectively independently selected from H atom or C1-C20 aliphatic straight-chain or branched-chain hydrocarbyl groups or aromatic groups; Ar is an aryl group; A is N atom or CH; and L is a single bond or is selected from C4-C10 aromatic rings or aromatic heterocyclic rings. The invention also relates to application of the compounds in organic electroluminescent devices especially as an electron transport material and / or light-emitting body material of an OLED (organic light-emitting diode).
Owner:KUNSHAN VISIONOX DISPLAY TECH +2
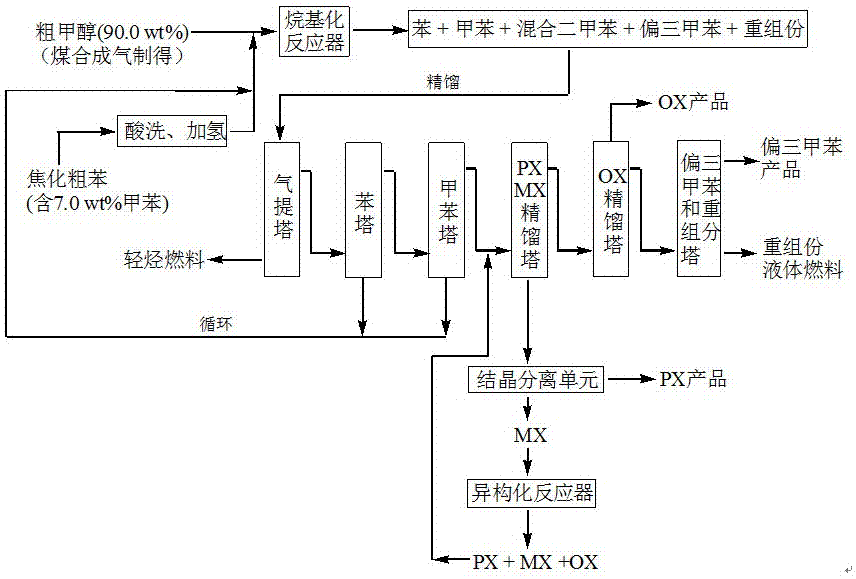
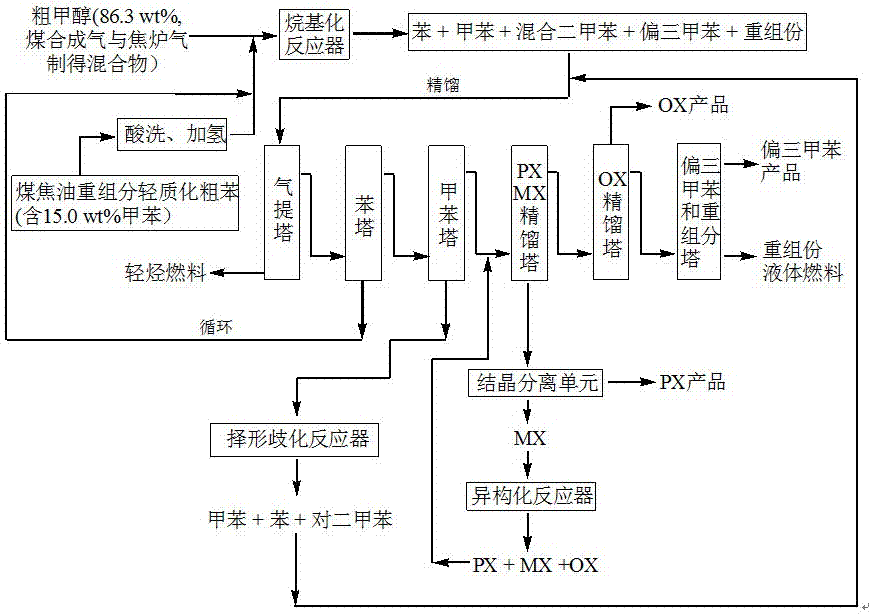
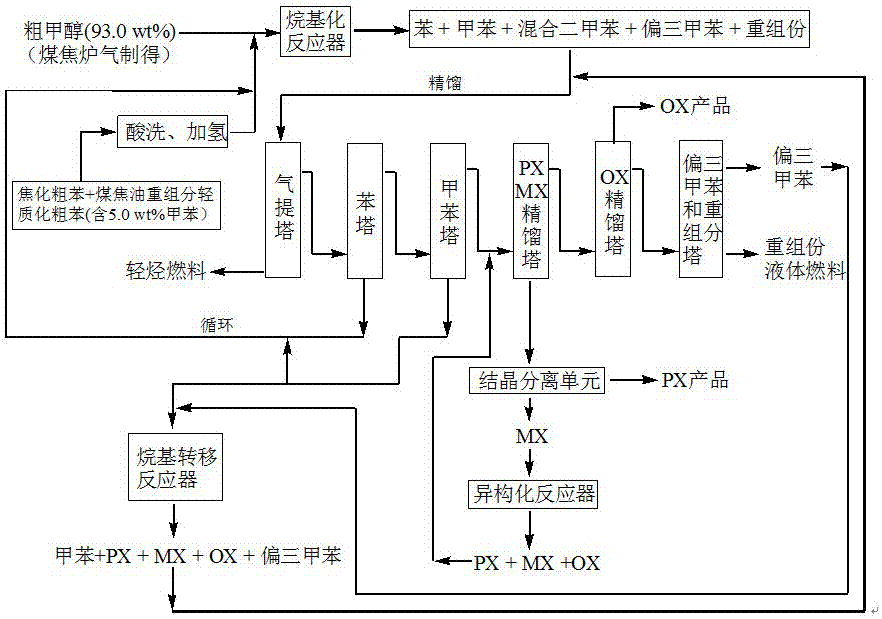
Method for producing p-xylene, o-xylene and 1,2,4-trimethylbenzene from coal-based raw materials
InactiveCN107473918AFlexible Adjustment StructureReduce coking and carbon depositionHydrocarbon by isomerisationMolecular sieve catalystsIsomerizationFixed bed
The invention relates to a method for producing p-xylene, o-xylene and 1,2,4-trimethylbenzene from coal-based raw materials. P-xylene, o-xylene and 1,2,4-trimethylbenzene are produced from raw materials including crude benzene and crude methanol by acid pickling, hydrogenation pretreatment, alkylation reaction, rectification, crystal separation and isomerization reaction by virtue of an alkylation catalyst having large outer specific surface area and mainly adopting weak acid and medium-strong acid as main components as well as an anti-coking alkylation reaction medium; the used catalyst is flake-like MCM-56, nanometer needle-like ZSM-5, flake-like MCM-49 or a nanometer Beta molecular sieve modified by two metal oxides or a compound thereof; and a novel special molecular sieve based catalyst is also used for isomerization, alkyl transfer and shape selective disproportionation reaction which are all carried out in a fixed bed reactor. By using the method, the production costs of p-xylene, o-xylene and 1,2,4-trimethylbenzene can be effectively reduced, and the aim of producing aromatic products according to a non-petroleum raw material route is thoroughly achieved.
Owner:TONGJI UNIV
Aluminum or aluminum alloy molten salt electroplating bath having good throwing power, electroplating method using the bath, and pretreatment method of the bath
The purpose of the present invention is to provide an electrical Al plating bath that poses little danger of exploding or igniting as a result of contacting air or water, and contains no benzene, toluene, xylene, naphthalene, or 1,3,5-trimethylbenzene, which have detrimental effects to humans. The present invention provides an electrical aluminum or aluminum alloy fused salt plating bath that is obtained by heat treatment of an electrical aluminum or aluminum alloy fused salt plating bath containing (A) a halogenated aluminum as the primary component and (B) at least one other type of halide after adding (C) one, two or more reducible compounds selected from the group consisting of hydrides of elements in Group 1 Periods 2 through 6 of the Periodic Table of Elements and / or hydrides of Group 13 Periods 2 through 6 of the Periodic Table of Elements and amine borane compounds.
Owner:DISPOL CHEMICALS CO LTD
Structure provided with zeolite separation membrane, method for producing same, method for separating mixed fluids and device for separating mixed fluids
InactiveUS20120000358A1Improve permeabilityEasy to separateMembranesSemi-permeable membranesRoom temperatureProduct gas
There is provided a zeolite separation membrane-provided article having gaps or pores larger than pores inherent to zeolite crystals and controlled within an appropriate range and being capable of achieving both high permeability and high separability for components with small difference in adsorption properties or a component having a smaller molecular diameter than the diameter of the pores, a method for producing the same, a method for separating mixed fluids, and a device for separating mixed fluids. The zeolite separation membrane-provided article is provided with a zeolite membrane having an N2 gas permeation speed at room temperature of 1.0×10−6 mol·m−2·s−1·Pa−1 or more and a permeation speed ratio of 1,3,5-trimethylbenzene / N2 at room temperature of 0.17 or more and being free from dyeing caused by the impregnation with Rhodamine B.
Owner:NGK INSULATORS LTD
Silica-based porous bulk for heat insulating material and coating-dry pressing preparation method thereof
InactiveCN101974314AHigh porosityHigh compressive strengthOther chemical processesHeat-exchange elementsPorosityCoated surface
The invention discloses a silica-based porous bulk for heat insulating material and a coating-dry pressing preparation method thereof. The preparation method comprises the following steps of: preparing porous silica powder through the hydrolytie polycondensation of tetraethoxysilane by using tetraethoxysilane as a precursor, a nonionic surfactant P123 as a template agent and trimethylbenzene as aswelling agent; hydro-thermally treating the silica powder so as to ensure that Si-OH in pore walls is completely polycondensed; coating the hydro-thermally treated silica powder with sol, and preparing the silica-based porous bulk for a heat insulating material by using the dry pressing method. The silica-based porous bulk is obtained by sintering the porous silica powder with oxide coated surface, wherein the oxide is alumina or titania. The mesoporous aperture of the silica-based porous bulk prepared by using the method is 21-35 nm, the mesoporous window is 10-18 nm, the total porosity is 65-82%, the mesoporosity is 80-99%, and the tensile strength is 45-180 MPa.
Owner:BEIHANG UNIV
Imine-bonded covalent organic framework
InactiveCN104927050ALarge specific surface areaHigh crystallinityHybrid capacitor electrodesCell electrodesVacuum pumpingSolvent
The invention discloses an imine-bonded covalent organic framework. A preparation method for the covalent organic framework comprises the following steps: adding 2,5-dipropargyl terephthalaldehyde and sym(4-aminophenyl) benzene into a solvent to obtain a mixture, and ultrasonically dispersing the mixture to form a suspension liquid, wherein the solvent is a mixed solvent of sym-trimethylbenzene, n-butyl alcohol and an acetic acid solution; carrying out liquid nitrogen freezing, vacuum-pumping and degassing on the suspension liquid, sealing the suspension liquid, and carrying out a microwave-assisted solvothermal reaction to obtain a crude product; and collecting insoluble substances after centrifuging the crude product, washing and drying the insoluble substances to obtain the imine-bonded covalent organic framework. The preparation method is free of pollution, gentle and simple in reaction conditions, simple in requirements on equipment, and suitable for large-scale industrial production.
Owner:SHANGHAI JIAO TONG UNIV
Method for modifying synthetic veratric alcohol
InactiveCN101033172AExcellent qualityExcellent yieldOrganic chemistryOrganic compound preparationAlcoholWittig reaction
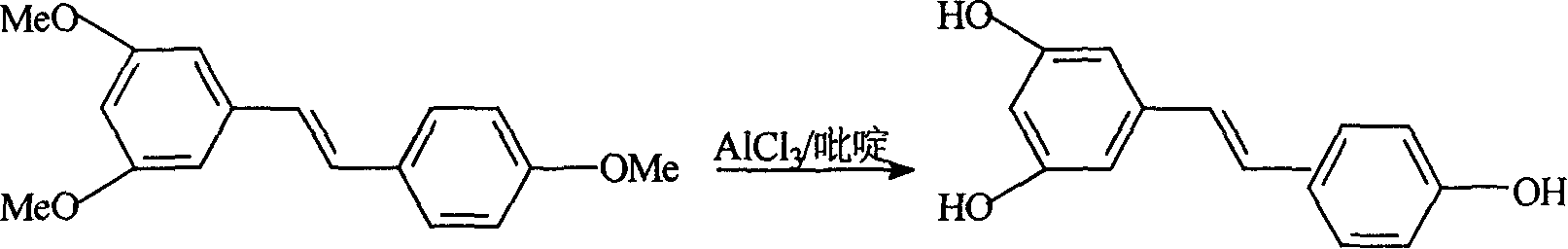
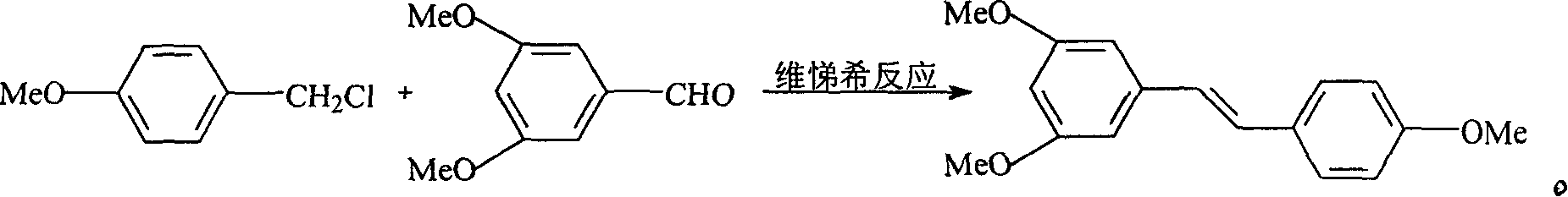
This invention relates to an improved method for synthesizing resveratrol, which takes 3, 5, 4'-methylate toluylene as the raw material, 1, 3, 5-trimethylbenzene as the solvent and hydroquinone as the antioxidant to synthesize resveratrol with alchlor / pyridine as the catalyst and applies commercial cheap anisyl chlorben and 3, 5-dimethoxy benzene formaldehyde for wittig reaction to prepare an intermediate 3, 5, 4'-methylate toluylene.
Owner:NANJING RALLY BIOCHEM
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

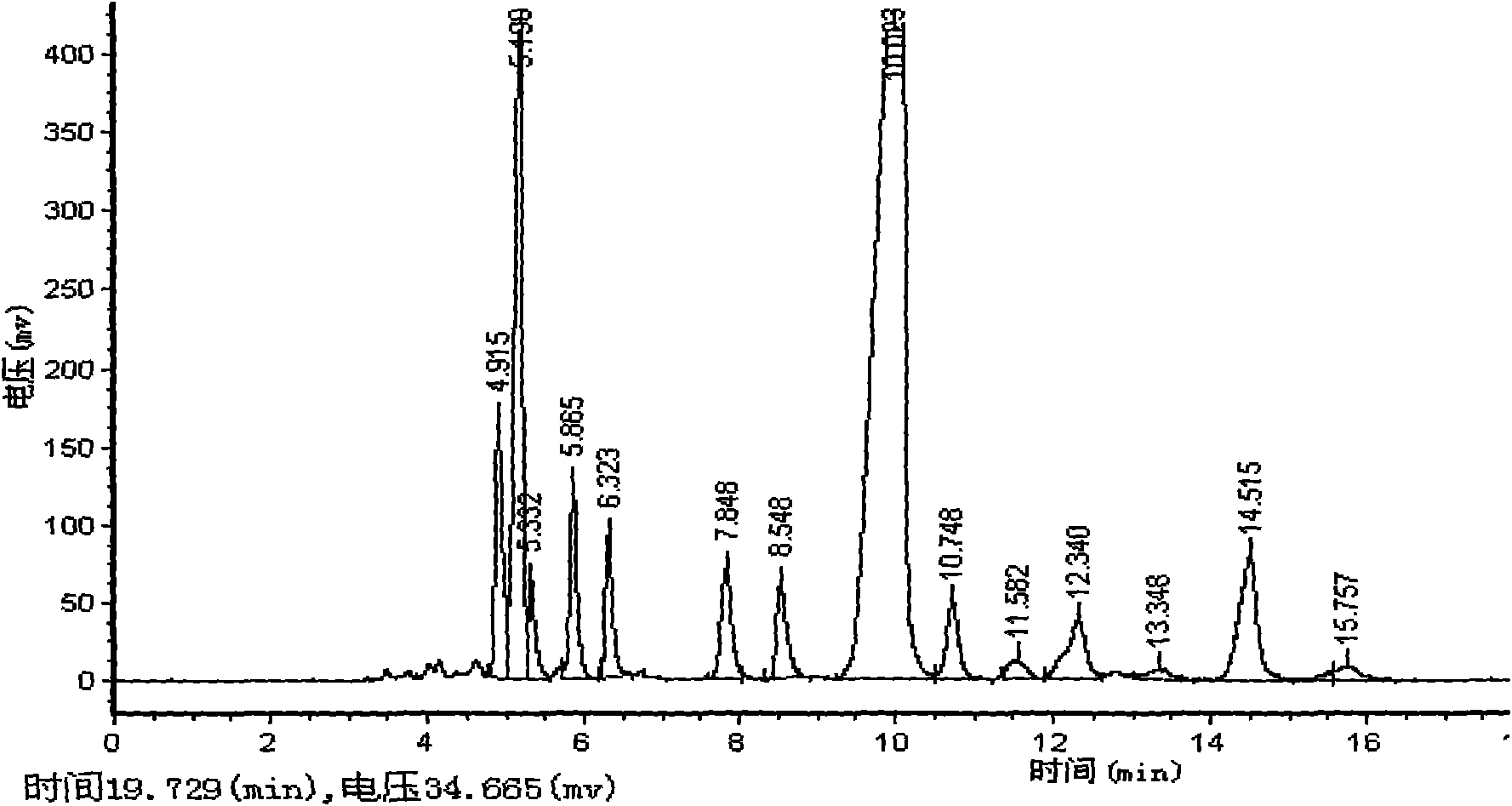
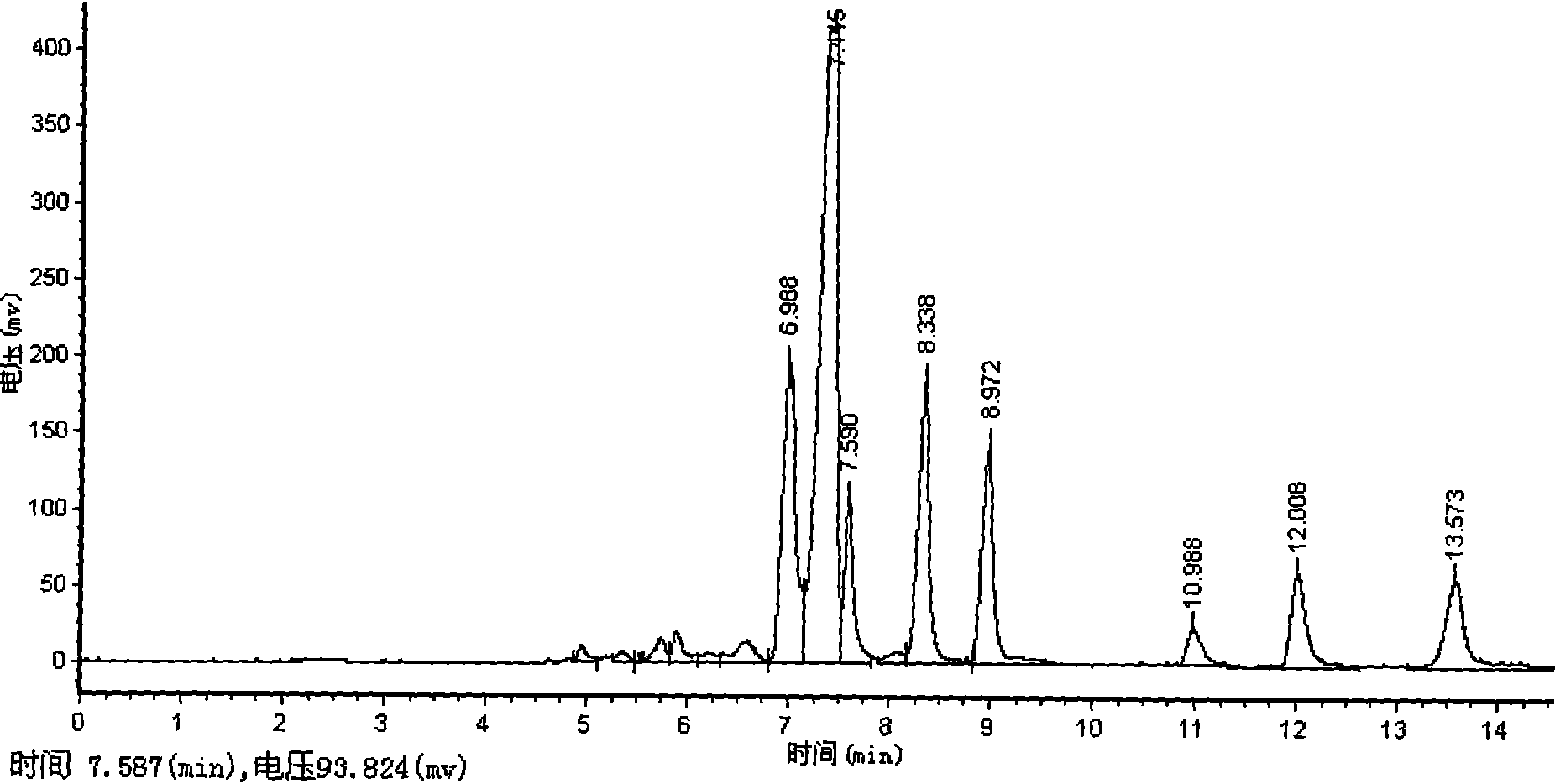
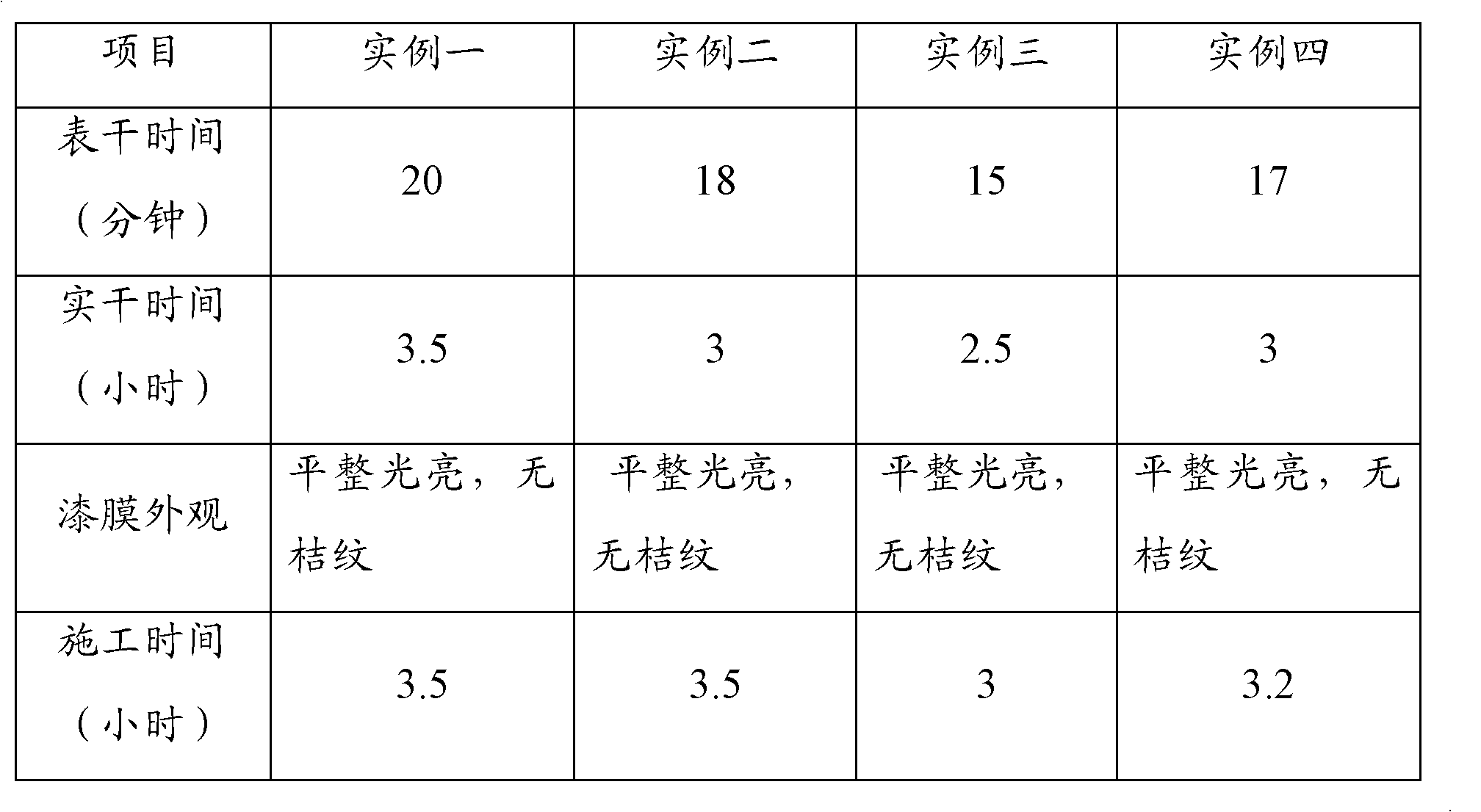
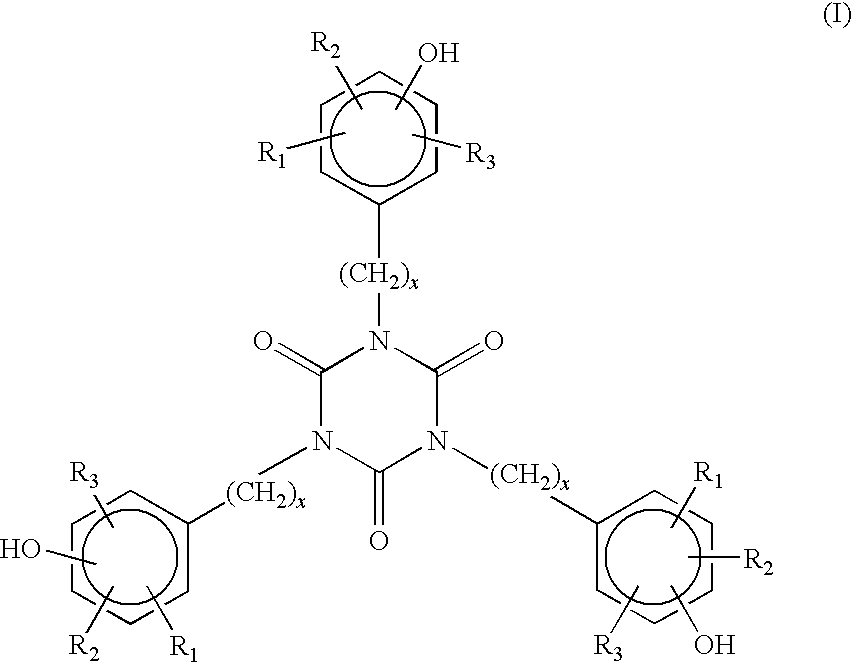



![6-trimethylphenyl-6H-6-boroheterobenzo[cd]pyrene derivatives and application thereof 6-trimethylphenyl-6H-6-boroheterobenzo[cd]pyrene derivatives and application thereof](https://images-eureka.patsnap.com/patent_img/8b6df69e-953c-4f2e-9750-2a81690176d6/HDA00002694090100011.png)
![6-trimethylphenyl-6H-6-boroheterobenzo[cd]pyrene derivatives and application thereof 6-trimethylphenyl-6H-6-boroheterobenzo[cd]pyrene derivatives and application thereof](https://images-eureka.patsnap.com/patent_img/8b6df69e-953c-4f2e-9750-2a81690176d6/HDA00002694090100012.png)
![6-trimethylphenyl-6H-6-boroheterobenzo[cd]pyrene derivatives and application thereof 6-trimethylphenyl-6H-6-boroheterobenzo[cd]pyrene derivatives and application thereof](https://images-eureka.patsnap.com/patent_img/8b6df69e-953c-4f2e-9750-2a81690176d6/HDA00002694090100021.png)