A sleep peptide fusion protein and its application
A technology of fusion protein and sleep peptide, which is applied in the field of sleep peptide fusion protein and its application, can solve the problems of difficulty in industrialization, high industrial cost of fusion protein, and difficulty in purification of small molecule proteins, and achieves the advantages of purification and simple purification steps High efficiency, reduced dosing frequency and dose effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: Cloning construction of HSA cDNA:
[0032] For the extracted human liver tissue mRNA, put 100mg of human liver tissue directly into a mortar, add a little liquid nitrogen, and grind quickly. After the tissue becomes soft, add a small amount of liquid nitrogen, and grind again. into a centrifuge tube and let stand at room temperature for 5 min. Centrifuge at 4°C (12000g) for 10min, and take the supernatant. Add 0.2ml of chloroform, close the cap of the centrifuge tube tightly, shake vigorously by hand for 15s, then place in ice bath for 15min, centrifuge at 4°C (12000g) for 15min, and store the RNA in the upper aqueous phase. Transfer the aqueous phase to another clean centrifuge tube, add 0.5ml of isopropanol, mix well, leave at room temperature for 10min to precipitate RNA, and then centrifuge at 4°C (12000g) for 10min. Remove the supernatant, add 1ml of 75% ethanol to wash the precipitate, vortex, and then centrifuge at 4°C (7500g) for 5min to recover ...
Embodiment 2
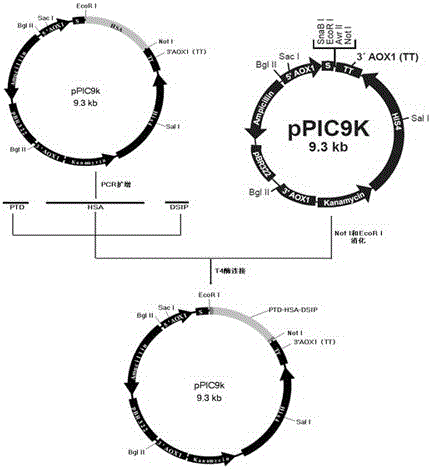
[0037] Example 2: Construction of PTD-HAS-DSIP (PHD) target gene:
[0038] Using the plasmid obtained in Example 1 as a template, the primers can be
[0039] P: 5'-GAATTCTACGGTCGTAAGAAAAGAAGACAACGTAGACGTGATGC
[0040] ACACAAGAGTGAG-3’
[0041] Under P: 5'- GCGGCCGCTTATTCTCCAGAAGCATCACCACCAGCCATAAGCCT
[0042] AAGGCAGCTTG-3'
[0043] The primers were synthesized by Shanghai Sangon Biotechnology Co., Ltd. The PCR method is as follows: add 2 μl of pPIC9K / H-T recombinant plasmid to 100 μl reaction system, 3 μl of 20 μmol / L upper and lower P primers, 2 mmol / L dNTP, 10 μl, 10 μl of 10× reaction buffer, pfu DNA polymerase 5U, (dNTP, reaction buffer, and pfu DNA polymerase are all products of Takara Company). The PCR conditions were denaturation at 94°C for 30 seconds, annealing at 50°C for 30 seconds, extension at 72°C for 3 minutes, and 30 cycles. The reaction product was analyzed by gel electrophoresis, and a band of expected size (about 2kb) was displayed. The PCR product PH...
Embodiment 3
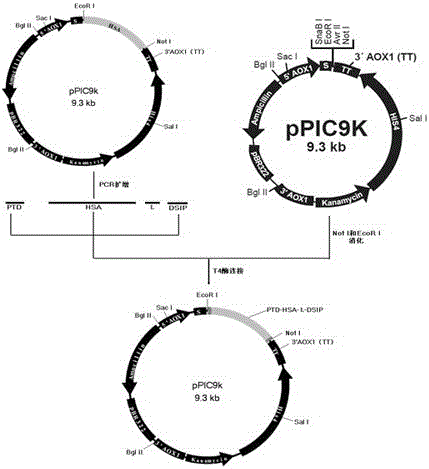
[0049] Embodiment 3: PTD-HAS-(Gly 4 Ser) 3 – Construction of DSIP (PHLD) target gene:
[0050] Using the plasmid obtained in Example 1 as a template, the primers can be
[0051] P: 5'-GAATTCTACGGTCGTAAGAAAAGAAGACAACGTAGACGTGATG
[0052] CACACAAGAGTGAG-3'
[0053] Lower P: 5'-GCGGCCGCTTATTCTCCAGAAGCATCACCACCAGCCCAAGAACC
[0054] TCCACCAGAACCTCCACCAGAACCTCCACCTAAGCCTAAGGCAGCTTG-3’
[0055] The primers were synthesized by Shanghai Sangon Biotechnology Co., Ltd. For PCR amplification, see Example 2 for the specific operation method.
[0056] For the gene sequence, please refer to SEQ ID NO: 2 in the description of the accompanying drawings. See the appendix for the plasmid construction process figure 2 .
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap