Novel human ependymin-like protein
a technology of pendymin and ependymin, applied in the field of human ependyminlike proteins, can solve the problems of short-term memory and long-term memory effect, and achieve the effect of improving the efficiency of the promoter
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Cloning of the cDNA Coding for Human Ependymin-Like Protein
[0421] From 5 .mu.g of human placental poly(A) .sup.+RNA (Clontech), a first strand DNA was synthesized using an oligo(dT) primer having XhoI site at a terminus and SuperScript II MMLV RNase H.sup.- reverse transcriptase (Gibco BRL). Then, using E. coli DNA polymerase I and RNase H, a second strand was synthesized. In this manner, a double-stranded cDNA was obtained from the poly(A).sup.- RNA. This double-stranded DNA was treated with Pfu DNA polymerase (Stratagene) to provide blunt ends and an EcoRI adapter was added. This double-stranded DNA with the EcoRI adapter added to either end was subjected to gel filtration to remove cDNAs about 1000 bp and less and using T4 polynucleotide kinase (Takara Shuzo), the EcoRI adapters were phosphorylated. This cDNA was then digested with XhoI, and after integration with .lambda.ZAPII EcoRI-XhoI arm (Stratagene), in vitro packaging was carried out to construct a human placental cDNA lib...
example 2
Northern Hybridization of Human Tissues
[0425] A membrane filter (MTN blot, Clonetech) preblotted with 2 .mu.g of human tissue poly(A).sup.+ RNA was subjected to prehybridization in a hybridization buffer (50% formamide, 5.times.SSPE (20.times.SSPE=3.6 M sodium chloride, 0.2 M sodium phosphate (pH 7.7), 20 mM EDTA), 5.times.Denhardt's solution, 0.1% SDS, 100 .mu.g / ml heat-denatured salmon sperm DNA) at 42.degree. C.
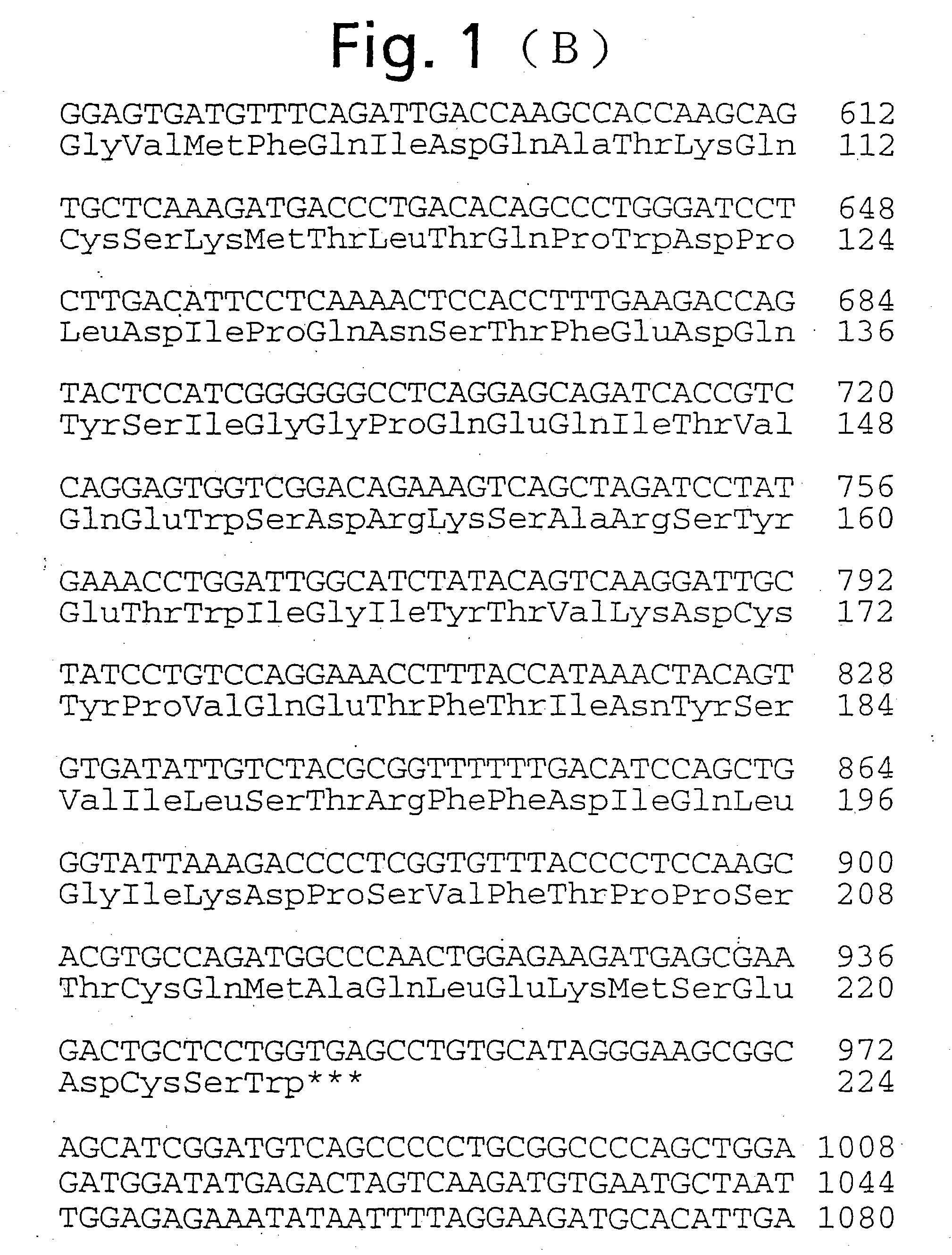
[0426] On the other hand, the BamHI-SpeI 383 bp cDNA fragment of the cDNA coding for human ependymin-like protein as shown in FIG. 1 (the cDNA fragment having a nucleotide sequence of bases no. 643 to no. 1025 of the base sequence shown in FIG. 1), as a probe, was labeled with [.alpha.-.sup.32P]dCTP (DuPont) using Randam Primer Labeling Kit (Amersham). After 12 hours of prehybridization in a hybridization buffer (50% formamide, 5.times.SSPE, 5.times.Denhardt's solution, 0.1% SDS, 100 .mu.g / ml heat-denatured salmon sperm DNA) at 42.degree. C., hybridization was carried out ...
example 3
[0427] Cloning of cDNA Encoding Rat Ependymin-Like Protein
[0428] From 8-week-old male Sprague-Dawley rats, the whole brain was enucleated and the total RNA was prepared by the guanidine-isothiocyanate method. Then, using the oligo(dT) span column (Pharmacia), a poly(A) .sup.+RNA fraction was obtained from the total RNA prepared above. From 5 .mu.g of this poly(A) .sup.+RNA, a first strand DNA was synthesized using NotI site-terminated an oligo(dT) primer having NotI site at a terminus and SuperScript II MMLV RNase H.sup.- reverse transcriptase (Gibco BRL). Then, using E. coli DNA polymerase I, E. coli DNA ligase, and RNase H, a second strand DNA was synthesized to provide a double-stranded cDNA from the poly(A) .sup.+RNA. This double-stranded DNA was treated with T4 DNA polymerase (Gibco BRL) to prepare blunt ends and an SalI adapter was added. This double-stranded DNA with the SalI adapter added to either end was cut with NotI and subjected to gel filtration to remove cDNAs of abou...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com