Applications of CAPE (Caffeic Acid Phenylethyl Ester) in culturing hematopoietic stem/progenitor cells in vitro
An in vitro culture and hematopoietic stem technology, applied in the field of in vitro culture of hematopoietic stem/progenitor cells, can solve the problems of reducing the value of applied research, inability to achieve large-scale expansion of hematopoietic stem/progenitor cells, and insufficient stability of structure and function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1: Isolation of human umbilical cord blood mononuclear cells and CD34 positive cells
[0044] Follow the steps below to isolate human cord blood mononuclear cells and CD34-positive cells:
[0045] (1) Isolation of human umbilical cord blood mononuclear cells (mononuclear cell, MNC)
[0046]a. Mixing and sedimentation: Mix a fresh anticoagulated human cord blood sample with PBS at a volume ratio of 1:1, then add 1 / 4 of the total volume of 0.5% methylcellulose, mix gently, and let it stand at room temperature. After settling down to a clear boundary, carefully aspirate the supernatant into a 50mL centrifuge tube, and centrifuge at room temperature and 1800rpm for 5 minutes.
[0047] b. Resuspension, gradient centrifugation: Discard the supernatant, add 5mL PBS to each tube to resuspend the cells, then take four 10mL centrifuge tubes, carefully add 5mL of human lymphocyte separation medium pre-equilibrated to room temperature, and then dissolve the cell suspension...
Embodiment 2
[0054] Example 2: Culture of human umbilical cord blood mononuclear cells and CD34 positive cells in vitro
[0055] Human umbilical cord blood mononuclear cells obtained above, CD34 + Cells were divided into 1×10 6 pcs / hole, 2×10 5 The density of each well was inoculated in a low-adsorption 24-well plate, and added to it containing 10ng / mL IL-3, 20ng / mL IL-6, 20ng / mL TPO, 25ng / mL SCF and 50ng / mL Flt-3L Serum-free Stemspan medium (Stemcell technologies, CAT09605 / 09600 / 09805 / 09800), then CAPE was dissolved and diluted with DMSO, and added to the medium of the experimental group at a volume ratio of 1:2000, where CAPE was in the medium of the experimental group The concentration of DMSO was 1 μg / mL, and the same amount of DMSO was added to the culture medium of the control group. Next, the two groups of cells were placed in 5% CO 2 The culture was carried out in a 37°C incubator with saturated humidity. During the culture process, the liquid was replenished or subcultured eve...
Embodiment 3
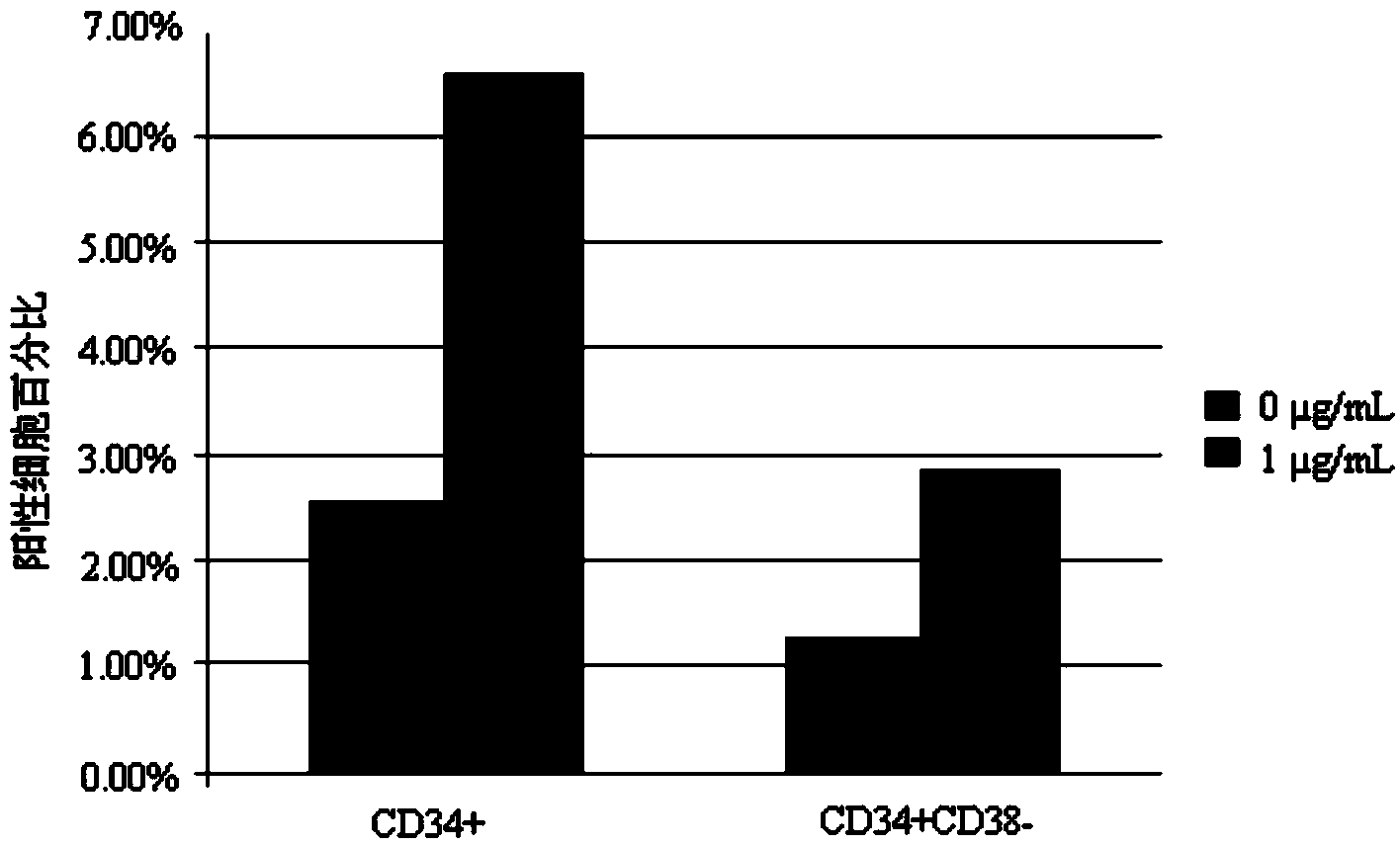
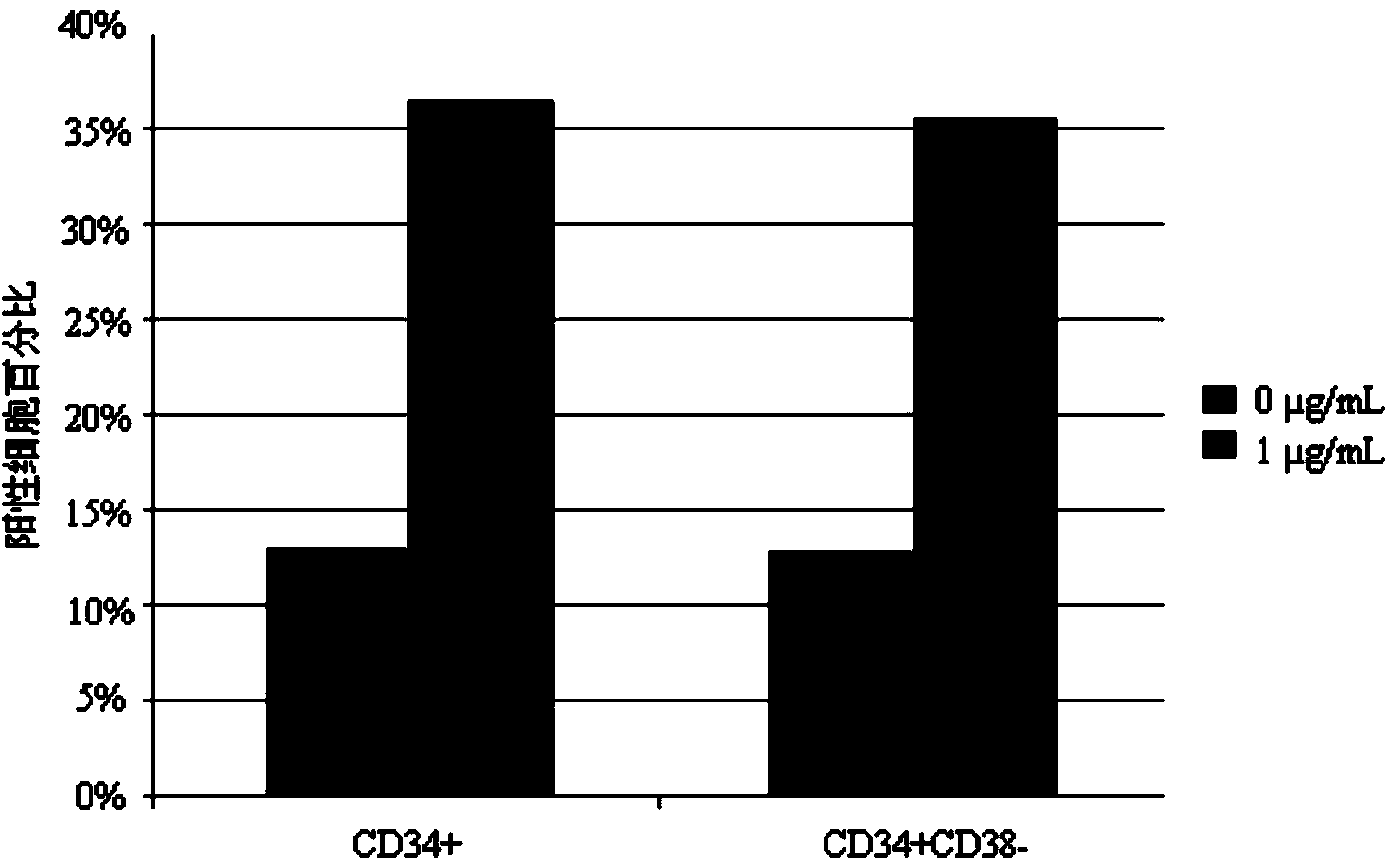
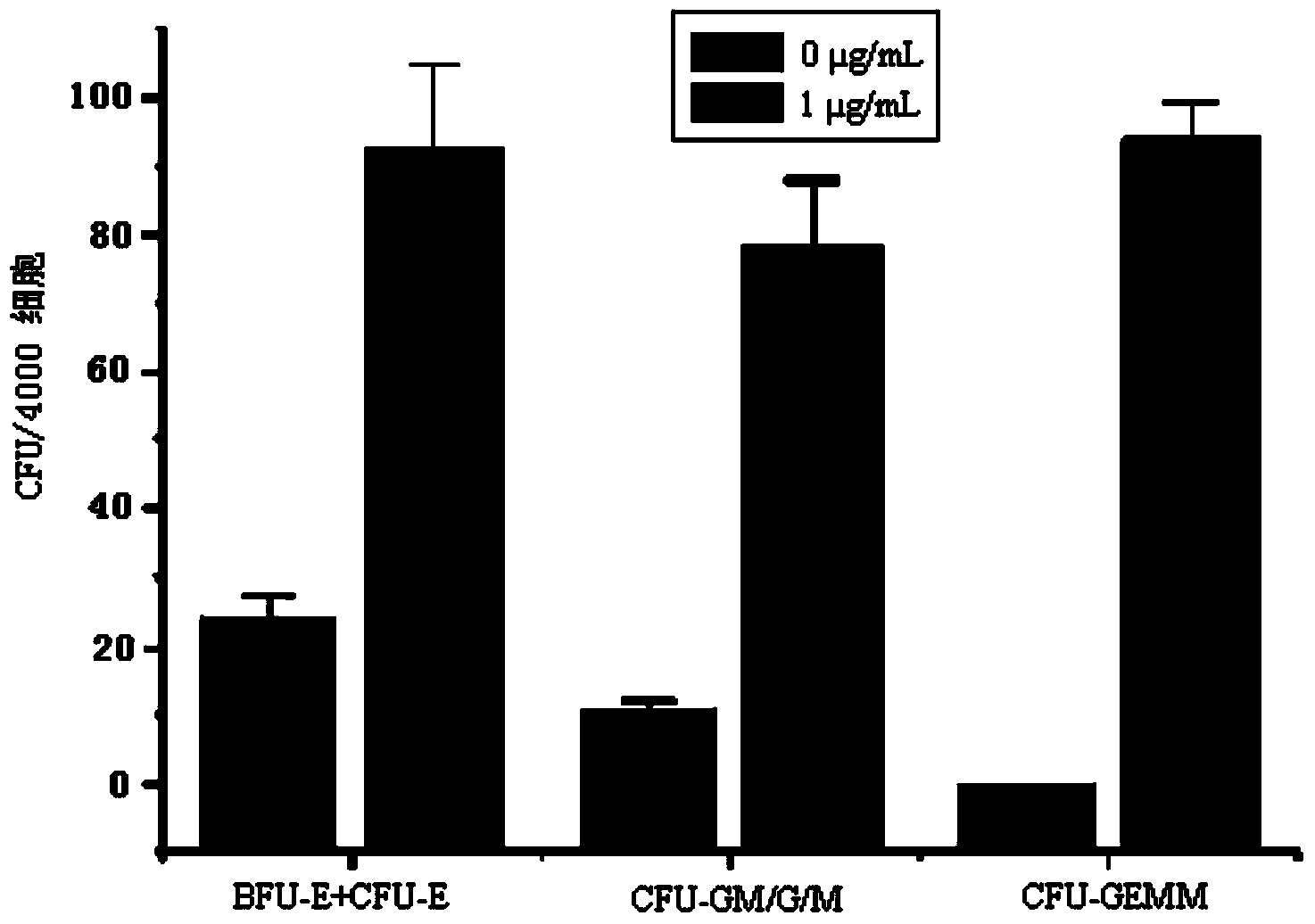
[0056] Example 3, detection of hematopoietic stem / progenitor cell expansion results
[0057] 3.1 Identification by flow cytometry
[0058] The human umbilical cord blood mononuclear cells or CD34-positive cells cultured for 7 days were detected by flow cytometry, and the detection indicators were the expression of surface markers CD34 and CD38. Specific steps are as follows:
[0059] Aspirate the cells in the well plate, put them into a 1.5mL EP tube, and fill up with PBS;
[0060] Centrifuge at 3000rpm for 5min;
[0061] Aspirate the supernatant and fill up with PBS again;
[0062] Centrifuge again at 3000rpm for 5min;
[0063] Aspirate the supernatant and add 100 μL of PBS;
[0064] Add 1 μL of anti-CD34 and CD38 antibodies to each tube of cells, seal the parafilm, put it on a rotating wheel in a refrigerator at 4°C, and incubate for 40 minutes;
[0065] Centrifuge at 3000rpm for 5min;
[0066] Wash twice with PBS, set the volume to 300-500 μL, and perform flow detect...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com