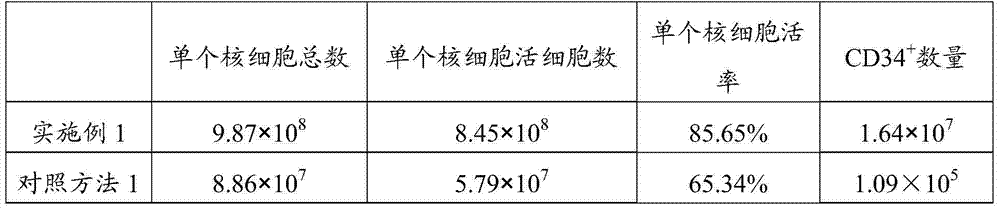
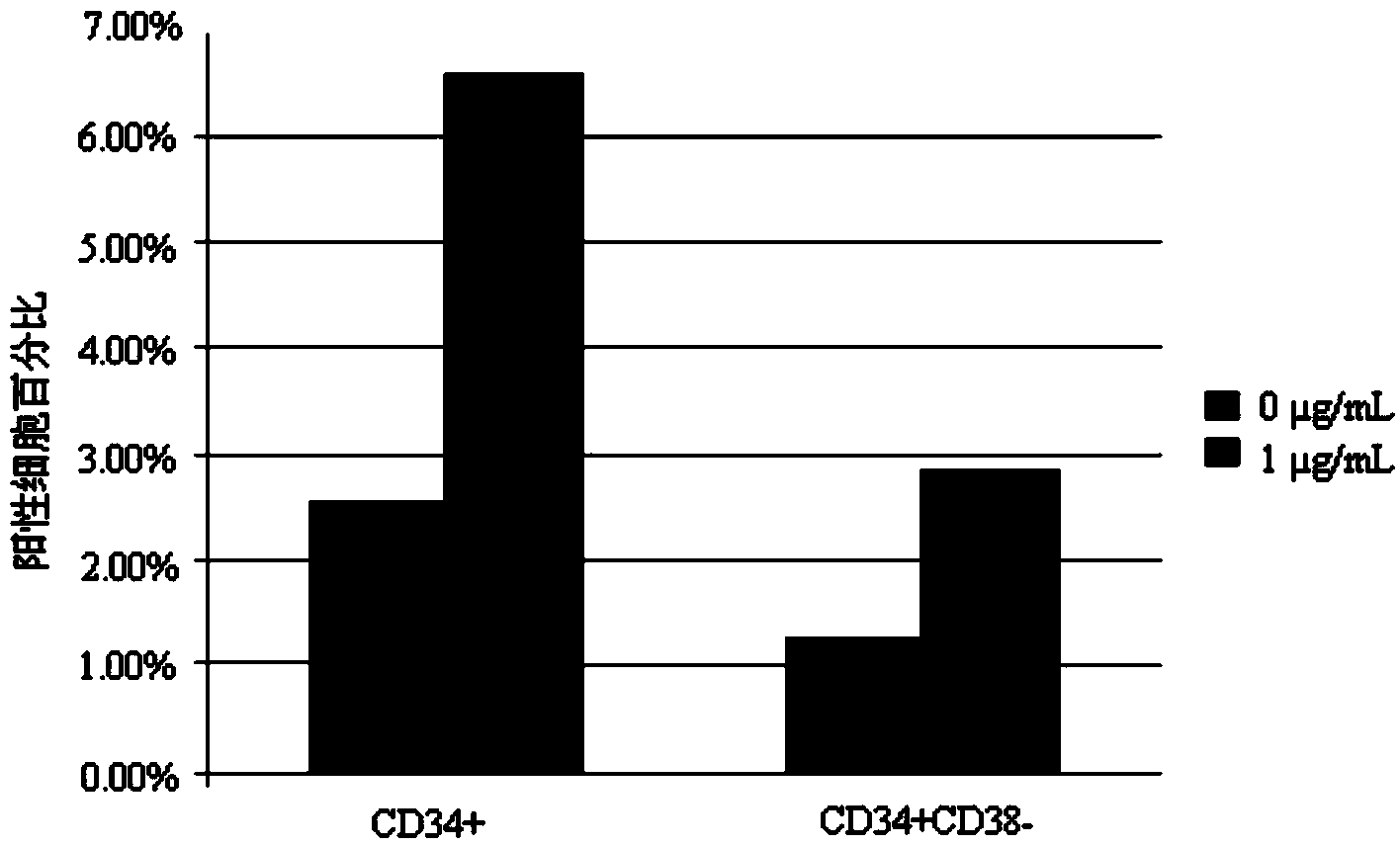
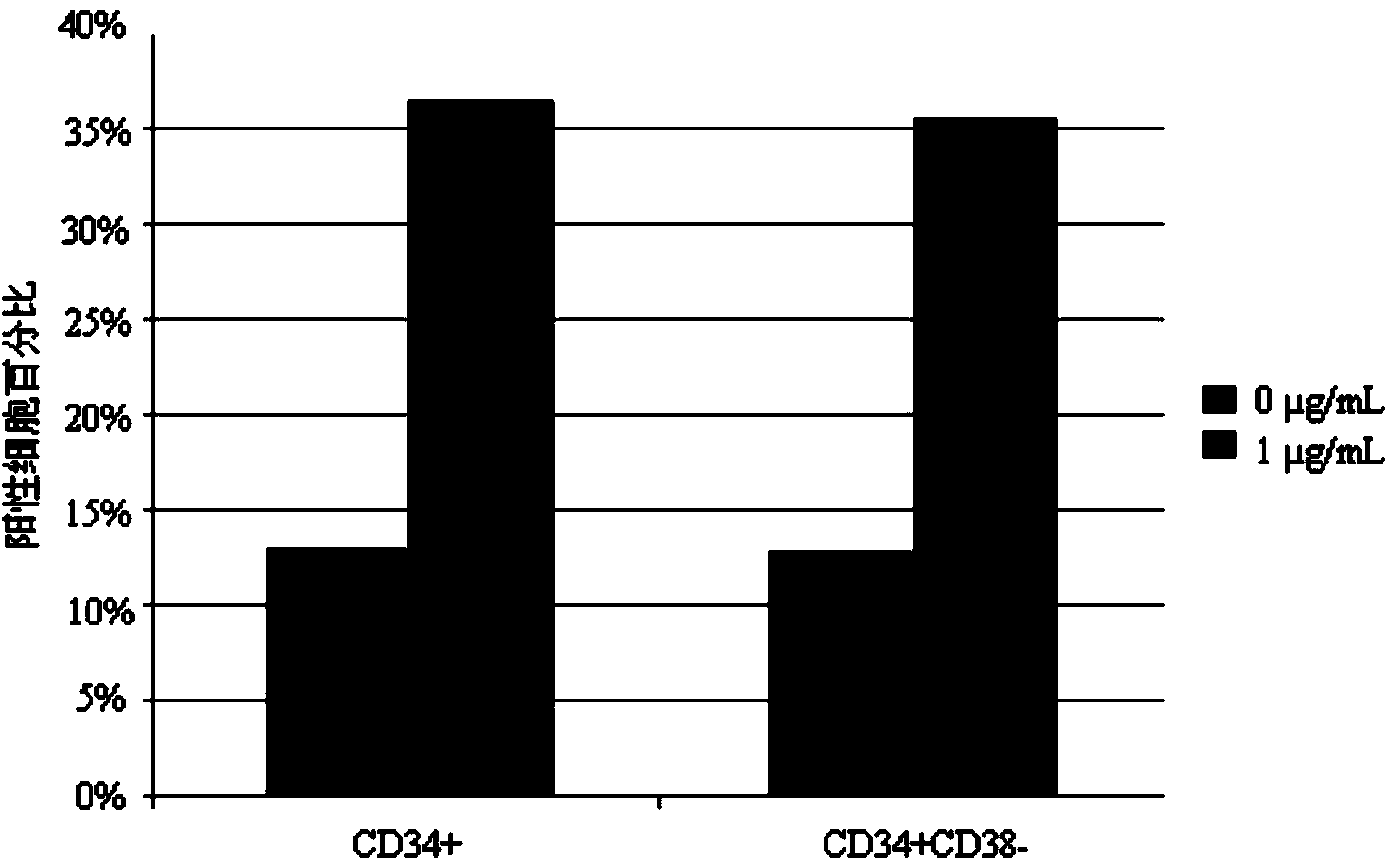
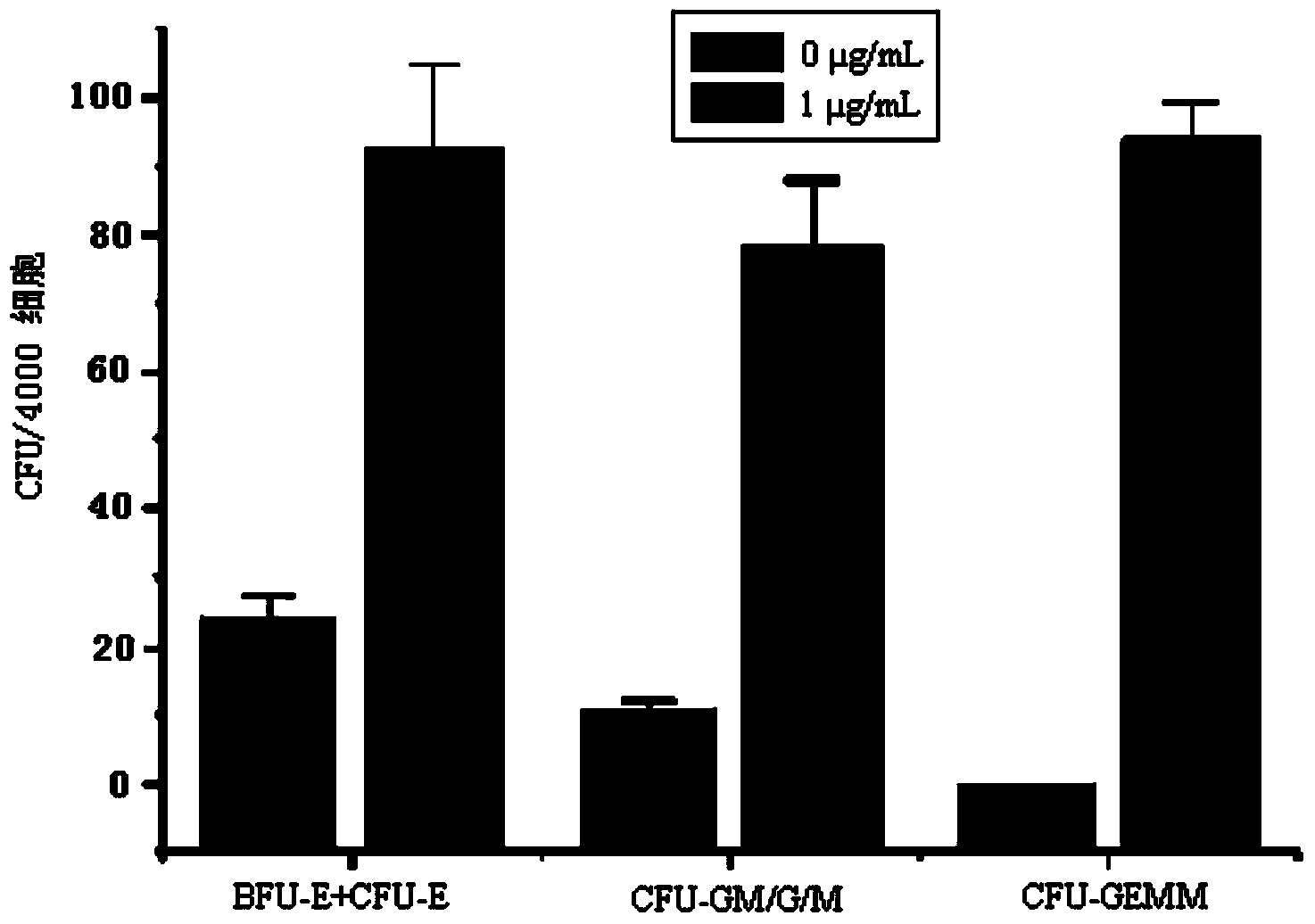
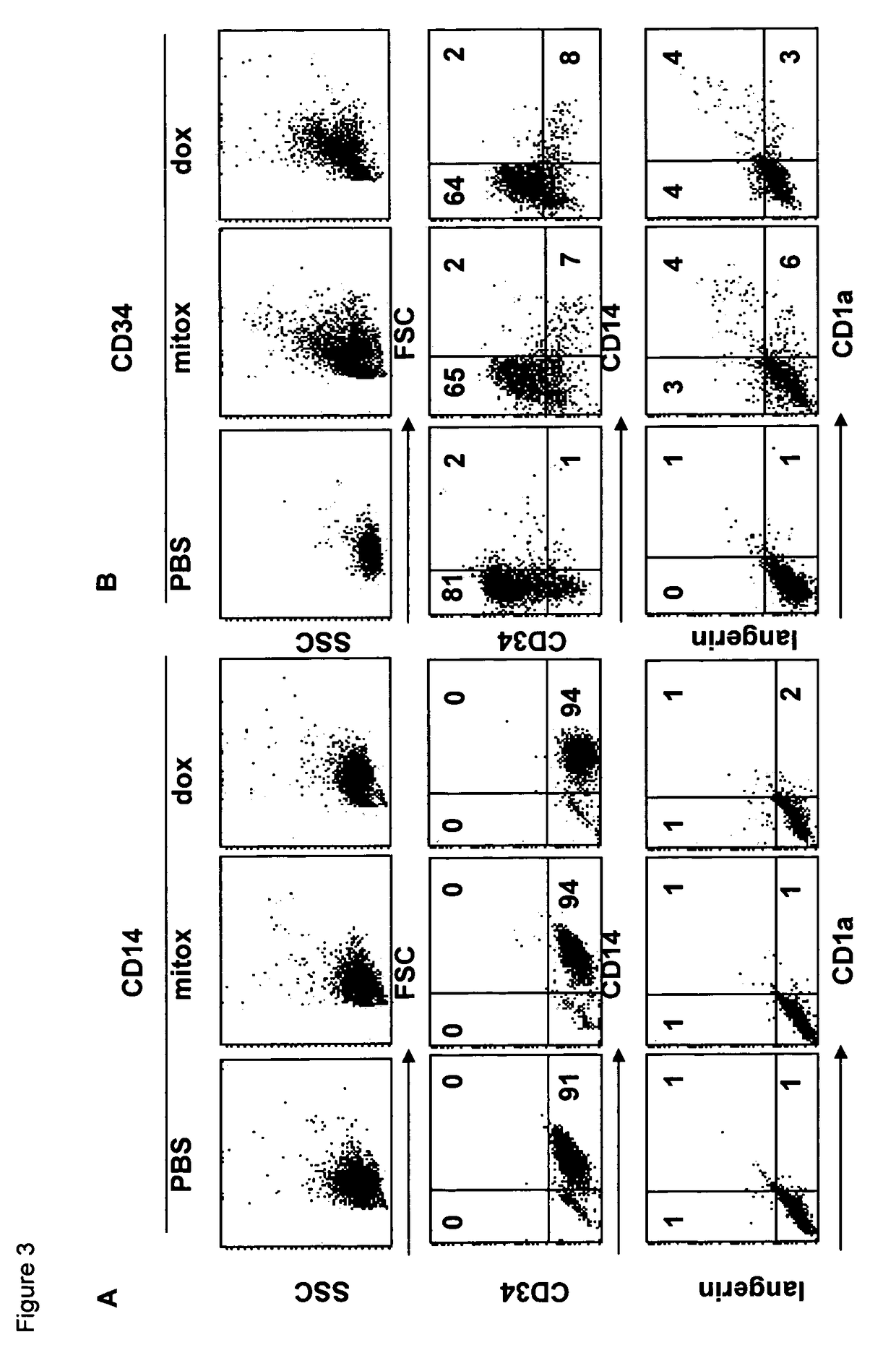
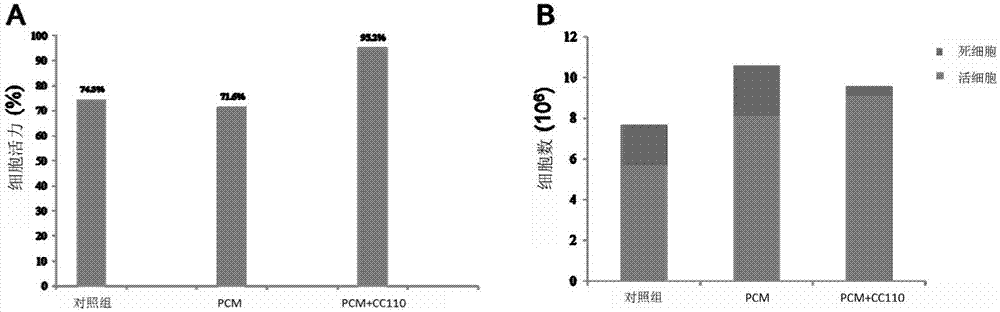
Patents
Literature
33 results about "Cd34 positive cell" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The CD34 antigen is expressed on hematopoietic stem and progenitor cells. This product can be used in place of the EasySep™ Human CD34 Positive Selection Kit (Catalog #18056) for even faster cell isolations.
Preparation method and preservation method of clinical application-level placental hematopoietic stem cells
InactiveCN103789262AIncrease contentEnhance cell viabilityDead animal preservationBlood/immune system cellsHydroxyethyl starchEnzyme digestion
The invention provides a preparation method of clinical application-level placental hematopoietic stem cells. The preparation method comprises the steps of pretreating a fresh delivered placenta immediately and then separating out umbilical arteries and umbilical veins, pouring a cleaning fluid into the chorionic vessels of the placenta and colleting the cleaning fluid after pouring, pouring an enzyme digestion fluid into the chorionic vessels of the placenta and digesting at 37 DEG C for 5-20min, pouring the collected fluid into the placenta and collecting the liquid after pouring, centrifuging the obtained fluid and re-suspending cells after removing the supernatant, and separating the resuspended cell suspension through a two-step process with hydroxyethyl starch and a lymphocyte separation medium to obtain the clinical application-level placental hematopoietic stem cells. The method is capable of increasing the number and the activity of the prepared placental hematopoietic stem cells and the content of CD34 positive cells, and the prepared placental hematopoietic stem cells are at the clinical application level and free of potential pathogenic contamination, and moreover, the method is low in preparation cost.
Owner:台州恩源生物科技有限公司
Method for preparing CD34 positive cells from umbilical cord mesenchymal stem cells
ActiveCN106434557ASuccessful separationSuccessfully and efficiently separateMammal material medical ingredientsSkeletal/connective tissue cellsMesenchymal stem cellUmbilical cord
The invention relates to a method for preparing CD34 positive cells from umbilical cord mesenchymal stem cells. The method specifically comprises the following steps: (a) providing mesenchymal stem cells from an umbilical cord; (b) performing primary culture on the mesenchymal stem cells; (c) performing subculture on the mesenchymal stem cells; (d) in a subculture process, adding an inductive agent in a culture medium for inducing the mesenchymal stem cells; (e) obtaining the CD34 positive cells. The invention also relates to cell products of the CD34 positive cells, prepared through the method and application of the cell products. The method disclosed by the invention has excellent technical effects described in the description.
Owner:BOYALIFE
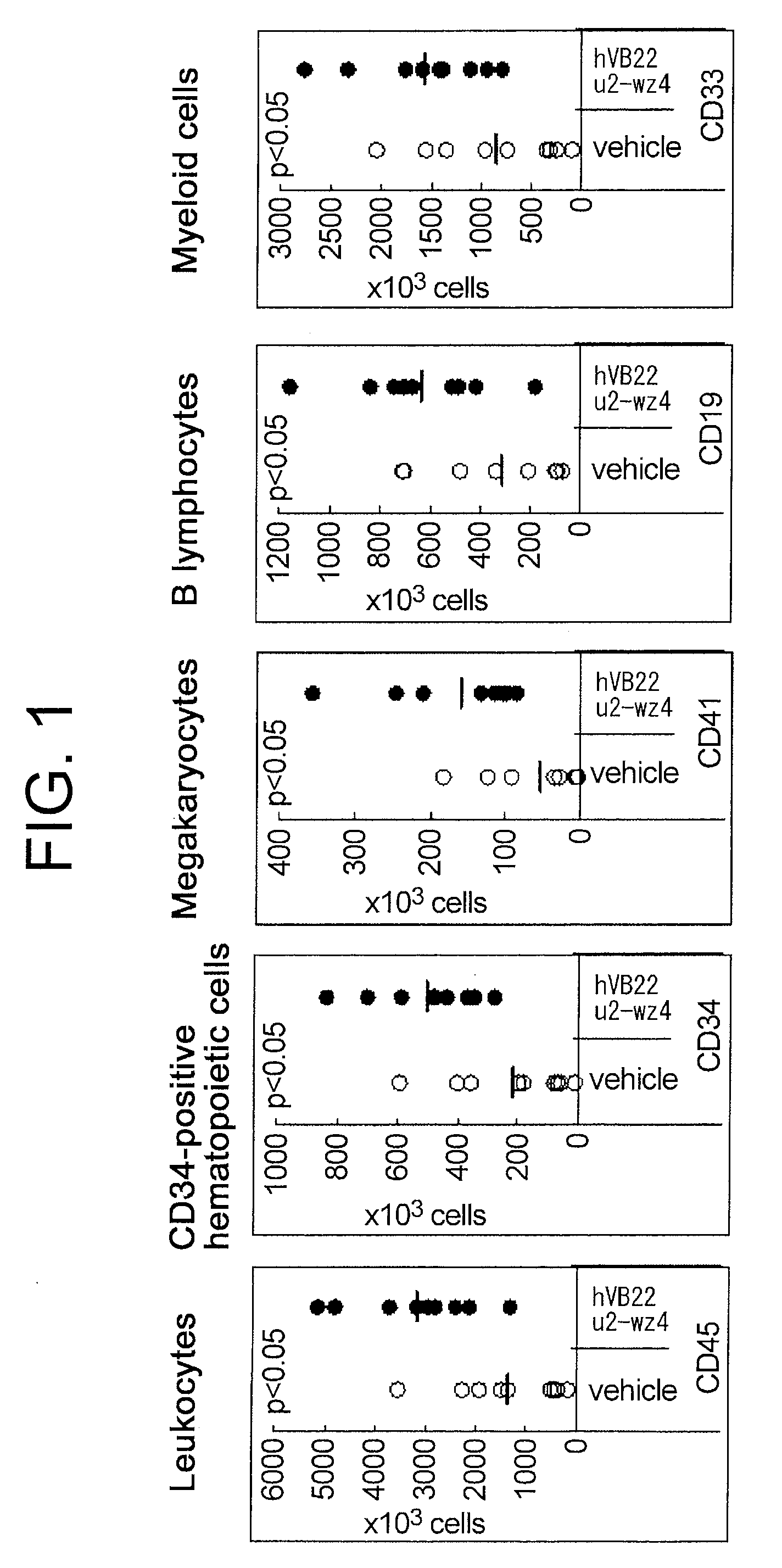
Agents for Promoting the Growth of Hematopoietic Stem Cells
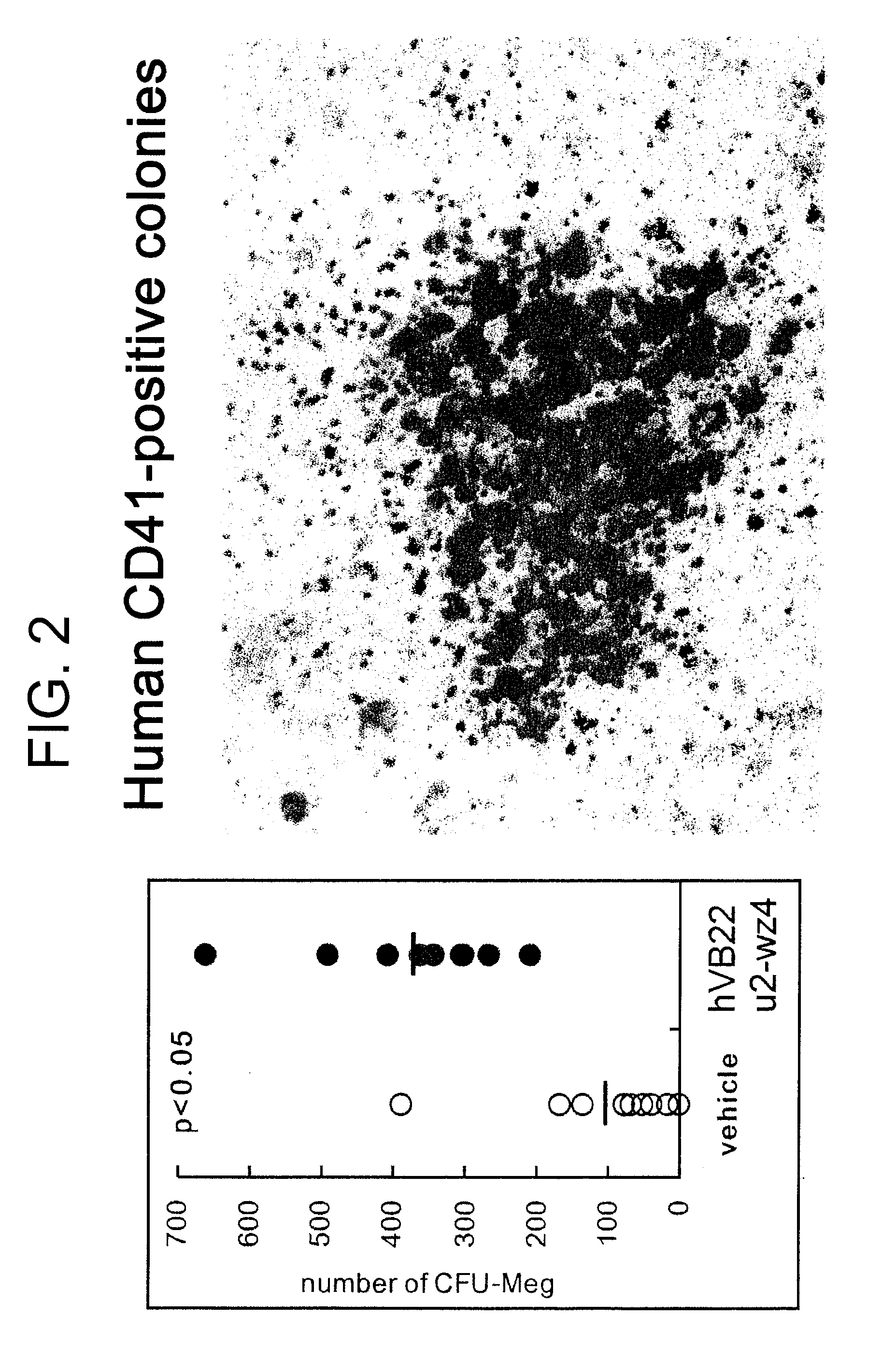
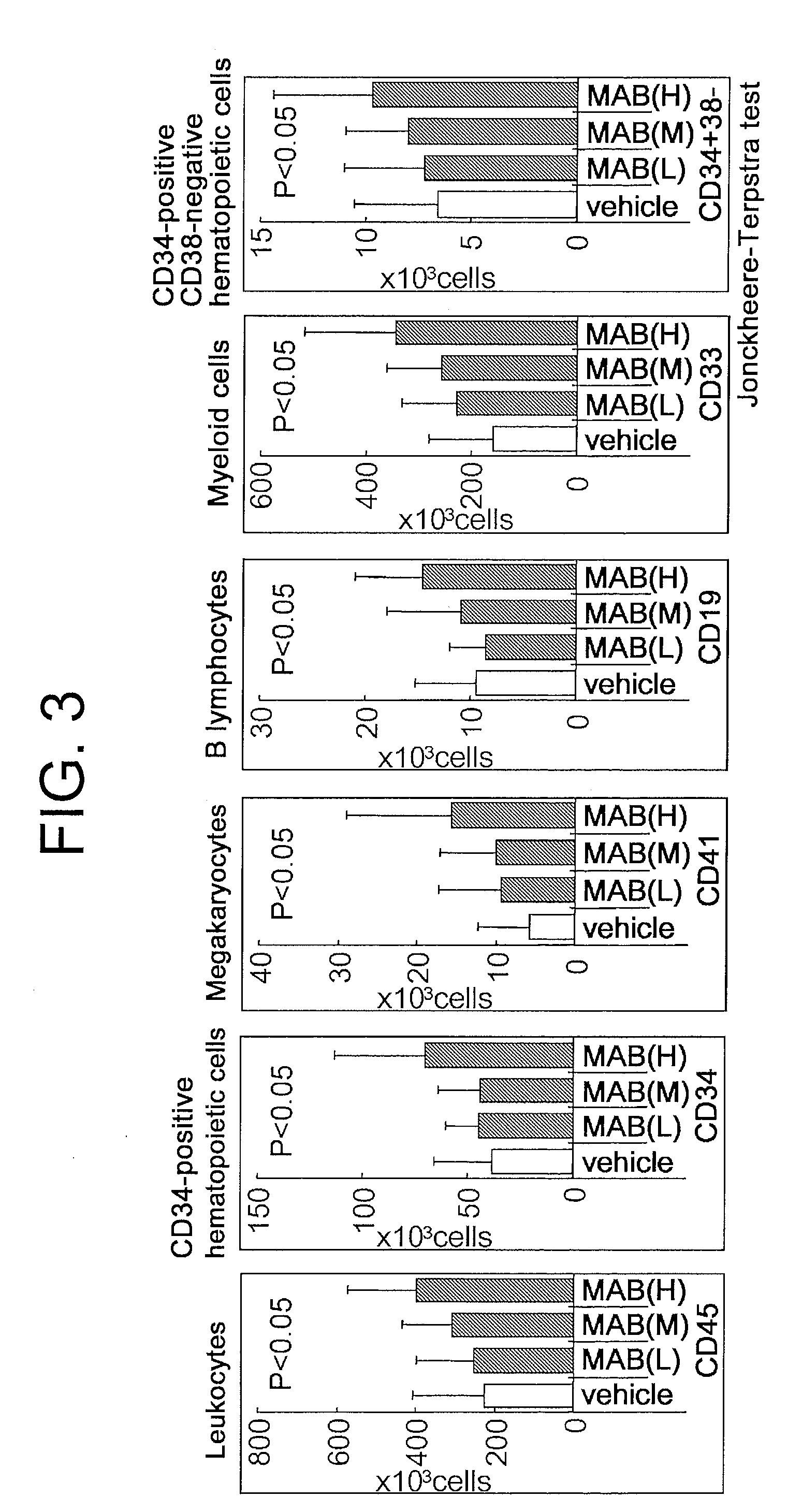
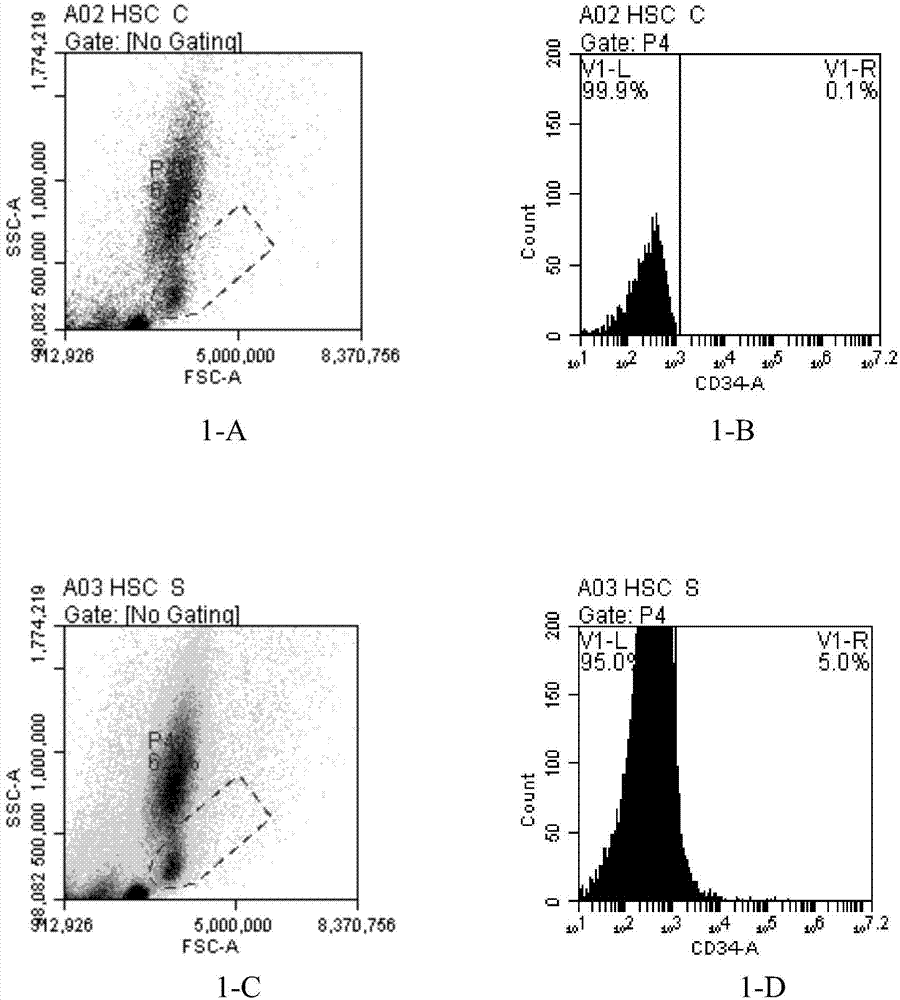
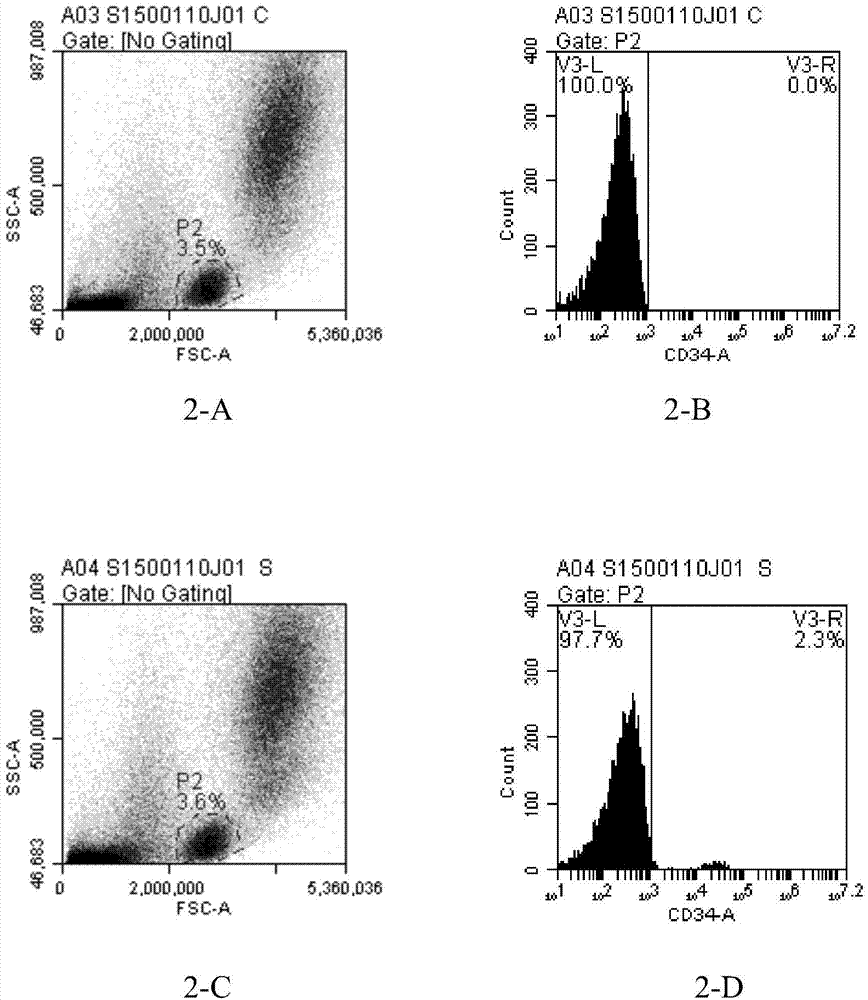
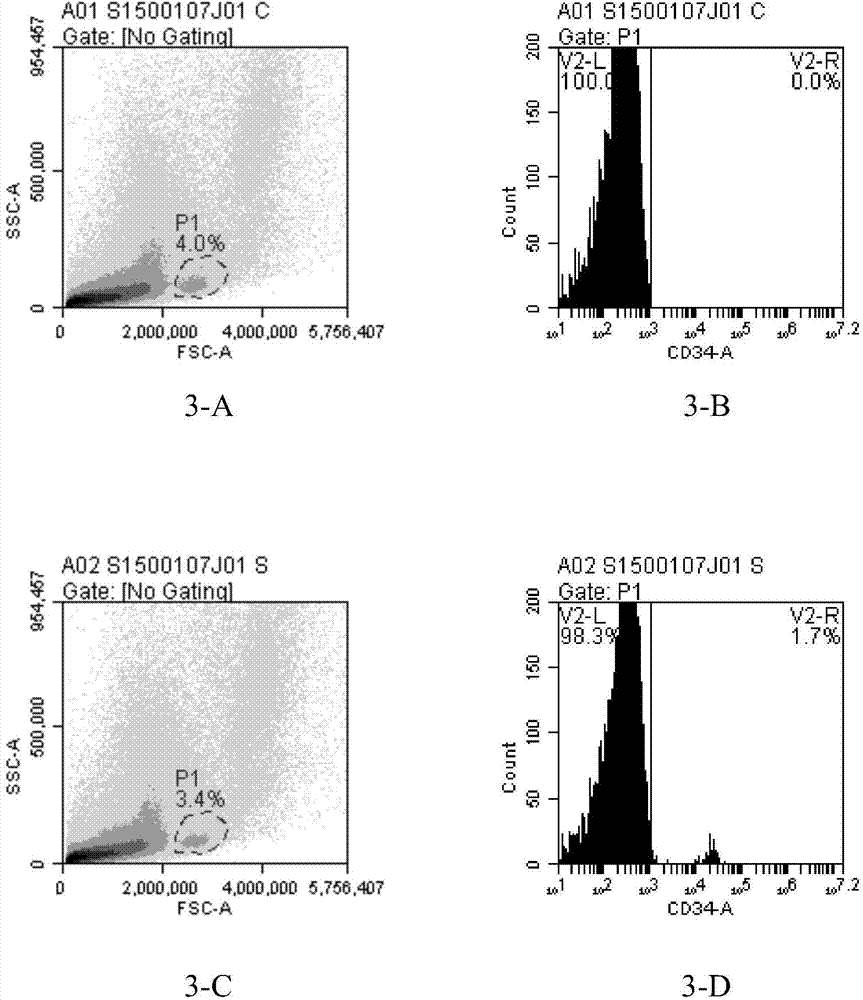
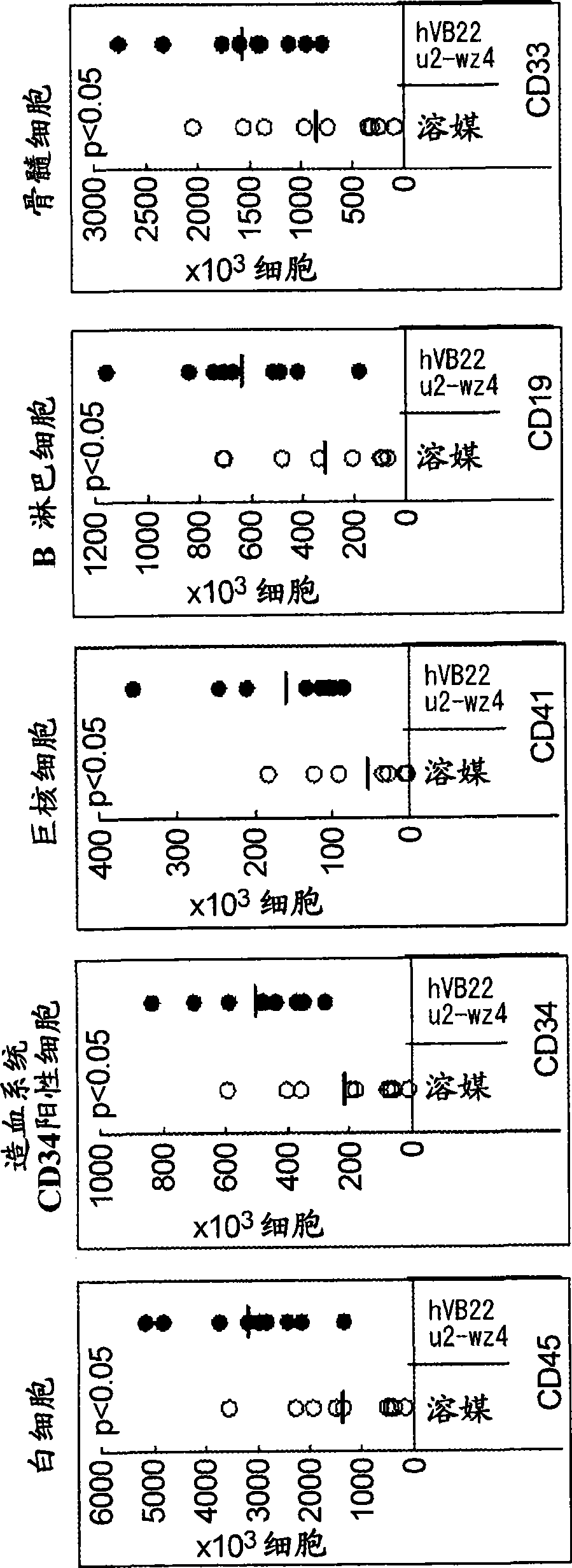
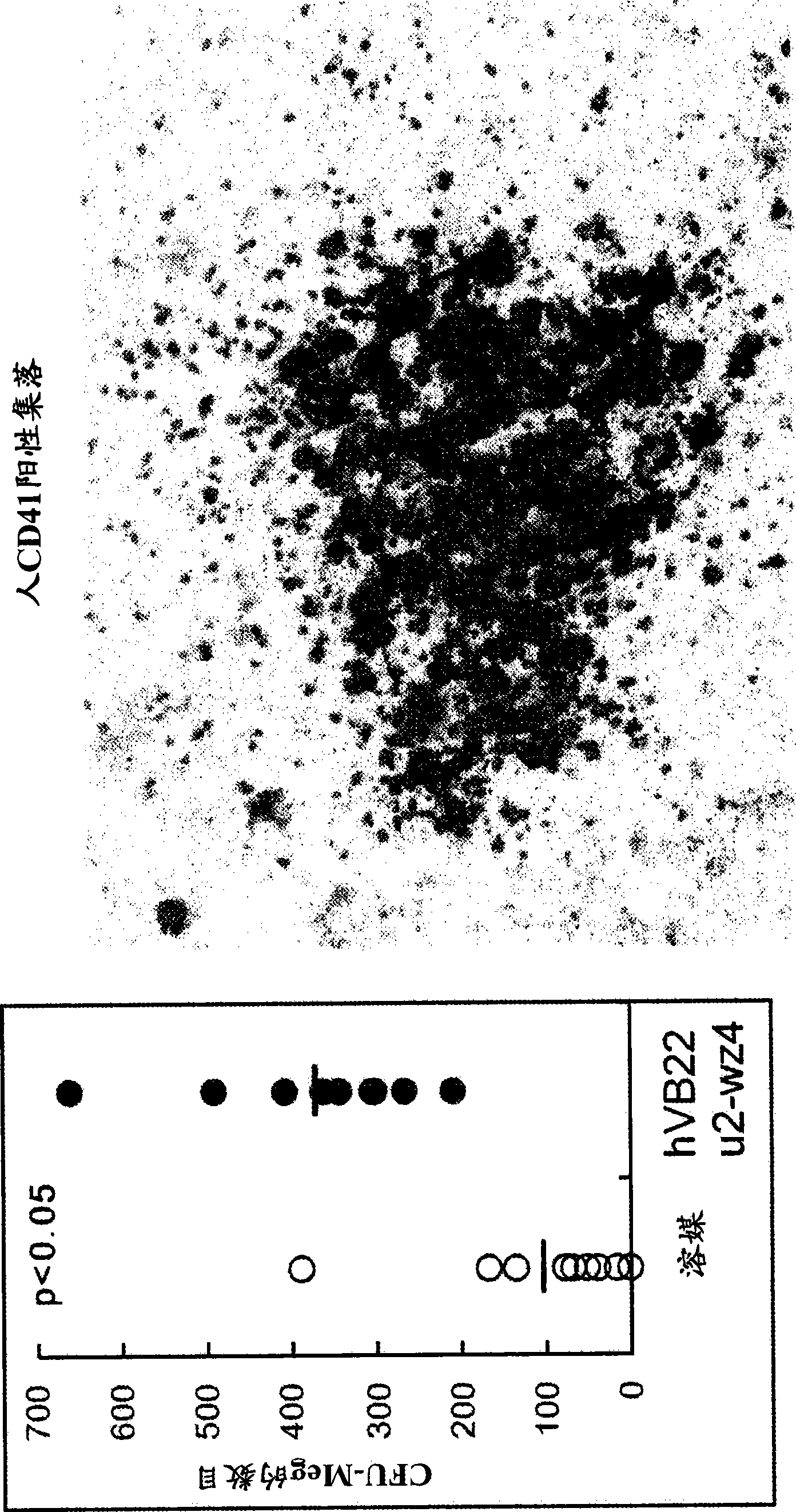
The present inventors discovered that the administration of an agonistic minibody (VB22B sc(Fv)2) against the TPO receptor resulted in not only the induction of human megakaryocyte-specific differentiation (increase in platelet precursor cells), but also the engraftment of transplanted hematopoietic stem cells derived from human cord blood (CD34-positive cells) and significant increase in multi-lineage hematopoietic precursor cells. TPO and TPO receptor agonists can be used as agents for promoting the growth of CD34-positive hematopoietic cells or agents for promoting the engraftment of transplanted cells in the bone marrow, which can be effective when administered alone (without using G-CSF and erythropoietin in combination) after hematopoietic stem cell transplantation (in particular, cord blood transplantation). Furthermore, TPO and TPO receptor agonists can be used as agents for promoting the growth and / or differentiation of multilineage hematopoietic precursor cells and agents for promoting the recovery of multilineage hematopoiesis.
Owner:CHUGAI PHARMA CO LTD
Preparation method of placenta hematopoietic stem cells
ActiveCN104711226AIncrease the number ofHigh activityBlood/immune system cellsVeinHydroxyethyl starch
The invention relates to the technical field of biology, and discloses a preparation method of placenta hematopoietic stem cells. The method comprises the following steps: cleaning a pretreated placenta by injection with an irrigating solution; injecting an enzymolysis solution from the placenta artery to the placenta vein until the enzymolysis solution flows out, tying the placenta artery and vein, and standing; loosening the placenta artery and vein, injecting the irrigating solution from the artery, collecting all the liquid at the vein, centrifugating, taking the cell precipitate, recovering and purifying single karyocytes comprising hematopoietic stem cells sequentially by a hydroxyethyl starch process and an erythrocyte cracking process, and resuspending to obtain the placenta hematopoietic stem cells. The method improves the enzymolysis injection technique, selects multiple mutually-suitable components to constitute the novel enzymolysis solution which is used in the placenta hematopoietic stem cell preparation process, can mobilize the hematopoietic stem cells inside the placenta to quickly enter the blood circulation system within a short time, and enhances the single karyocyte quantity comprising hematopoietic stem cells, the living cell quantity and the CD34 positive cell quantity.
Owner:GUANGZHOU SALIAI STEMCELL SCI & TECH CO LTD
Method for extracting hemopoietic stem cells from placentas
The invention provides a method for extracting hemopoietic stem cells from placentas, and especially relates to a method for extracting hemopoietic stem cells from placentas in a lavage manner. In one embodiment of the invention, the method comprises the following steps: 1, collecting the placentas; 2, carrying out initial inspection on the placentas; 3, pre-disinfecting the placentas; 4, detecting the placentas and maternal blood; 5, lavaging hemopoietic stem cells of the placentas; and 6, concentrating and purifying the hemopoietic stem cells. The method has the advantages presented in the specification, and a flow cytometer detection result of the hemopoietic stem cells obtained through the method shows that the hemopoietic stem cells have a very high percentage of CD34 positive cells.
Owner:BOYALIFE
Applications of CAPE (Caffeic Acid Phenylethyl Ester) in culturing hematopoietic stem/progenitor cells in vitro
The invention relates to applications of CAPE (Caffeic Acid Phenylethyl Ester) in culturing hematopoietic stem / progenitor cells in vitro. The CAPE with certain concentration is added in an in-vitro hematopoietic stem / progenitor cell expansion system, the ratio of the expanded human umbilical cord blood mononuclear cells or expanded hematopoietic stem / progenitor cells in CD34 positive cells is obviously increased, the total colony number is remarkably increased, so that the in-vitro expansion of the hematopoietic stem / progenitor cells can be effectively promoted, and the in-vitro expansion efficiency of the hematopoietic stem / progenitor cells can be enhanced.
Owner:FIELD OPERATION BLOOD TRANSFUSION INST OF PLA SCI ACAD OF MILITARY
Preparation method for placenta hemopoietic stem cell
ActiveCN104774806AIncrease the number ofHigh activityBlood/immune system cellsHydroxyethyl starchRed blood cell
The invention relates to the biotechnical field and discloses a preparation method for a placenta hemopoietic stem cell. The preparation method comprises the following steps: irrigating and cleaning a pre-processed placenta with an irrigating solution; irrigating a mobilizing agent from placental arteries until the mobilizing agent stops flowing from the placental veins and the placental arteries and veins are ligatured and stood; loosening the placental arteries and veins and grouting the same amount of mobilizing agent from the arteries, collecting all liquids from the veins, and centrifuging to get cell sediments; then, recycling and purifying single karyocyte comprising the hemopoietic stem cell through a hydroxyethyl starch process and a red blood cell lysis process in sequence, and re-suspending to obtain the placenta hemopoietic stem cell. According to the preparation method, the mobilizing agent irrigating process is improved, a plurality of mutually suitable components are selected to form a novel mobilizing agent for being applied to a preparation process of the placenta hemopoietic stem cell, the placenta hemopoietic stem cell inside the placenta can be mobilized and can quickly enter a blood circulating system, and the quantity of single karyocyte comprising the hemopoietic stem cell as well as quantity of living cells of the single karyocyte and quantity of CD34 positive cells is increased.
Owner:GUANGZHOU SALIAI STEMCELL SCI & TECH CO LTD
Hematopoietic stem cell proliferation promoter
The present inventors discovered that the administration of an agonistic minibody (VB22B sc(Fv)2) against the TPO receptor resulted in not only the induction of human megakaryocyte-specific differentiation (increase in platelet precursor cells), but also the engraftment of transplanted hematopoietic stem cells derived from human cord blood (CD34-positive cells) and significant increase in multi-lineage hematopoietic precursor cells. TPO and TPO receptor agonists can be used as agents for promoting the growth of CD34-positive hematopoietic cells or agents for promoting the engraftment of transplanted cells in the bone marrow, which can be effective when administered alone (without using G-CSF and erythropoietin in combination) after hematopoietic stem cell transplantation (in particular, cord blood transplantation). Furthermore, TPO and TPO receptor agonists can be used as agents for promoting the growth and / or differentiation of multilineage hematopoietic precursor cells and agents for promoting the recovery of multilineage hematopoiesis.
Owner:CHUGAI PHARMA CO LTD
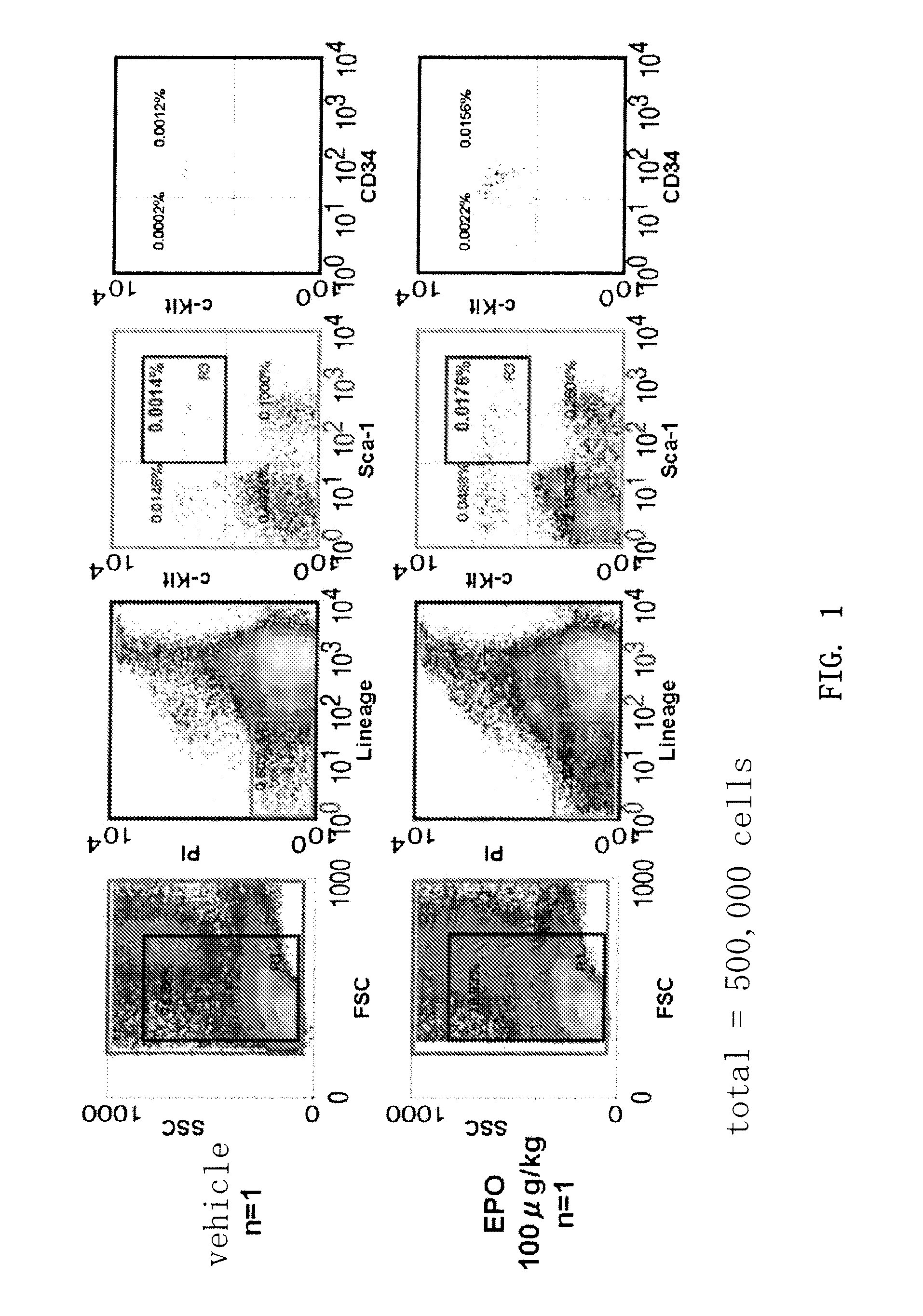
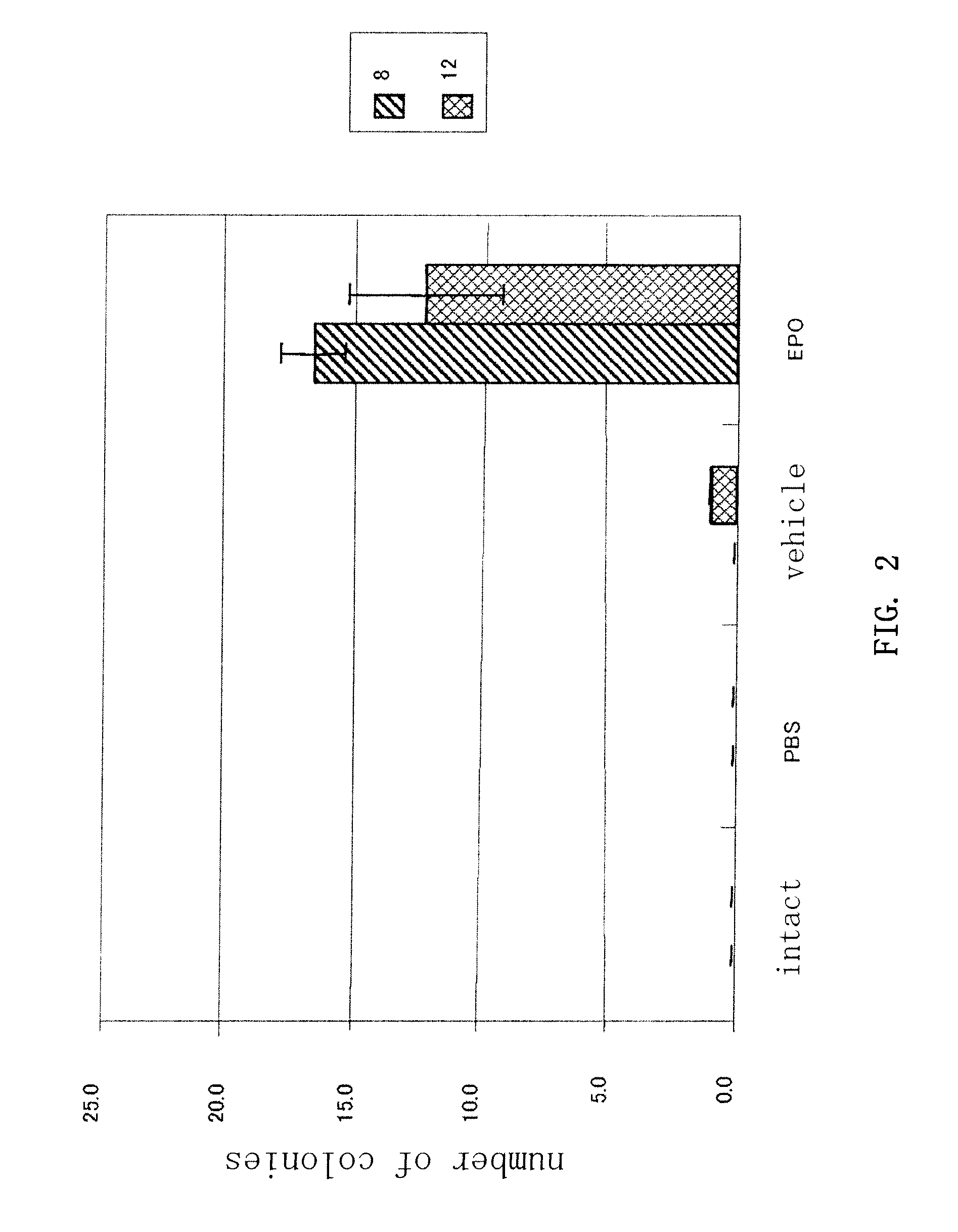
Treatment of Ischemic Diseases Using Erythropoietin
InactiveUS20090280094A1Useful in treatmentAntibacterial agentsBiocidePeripheral Vascular DisorderRevascularization
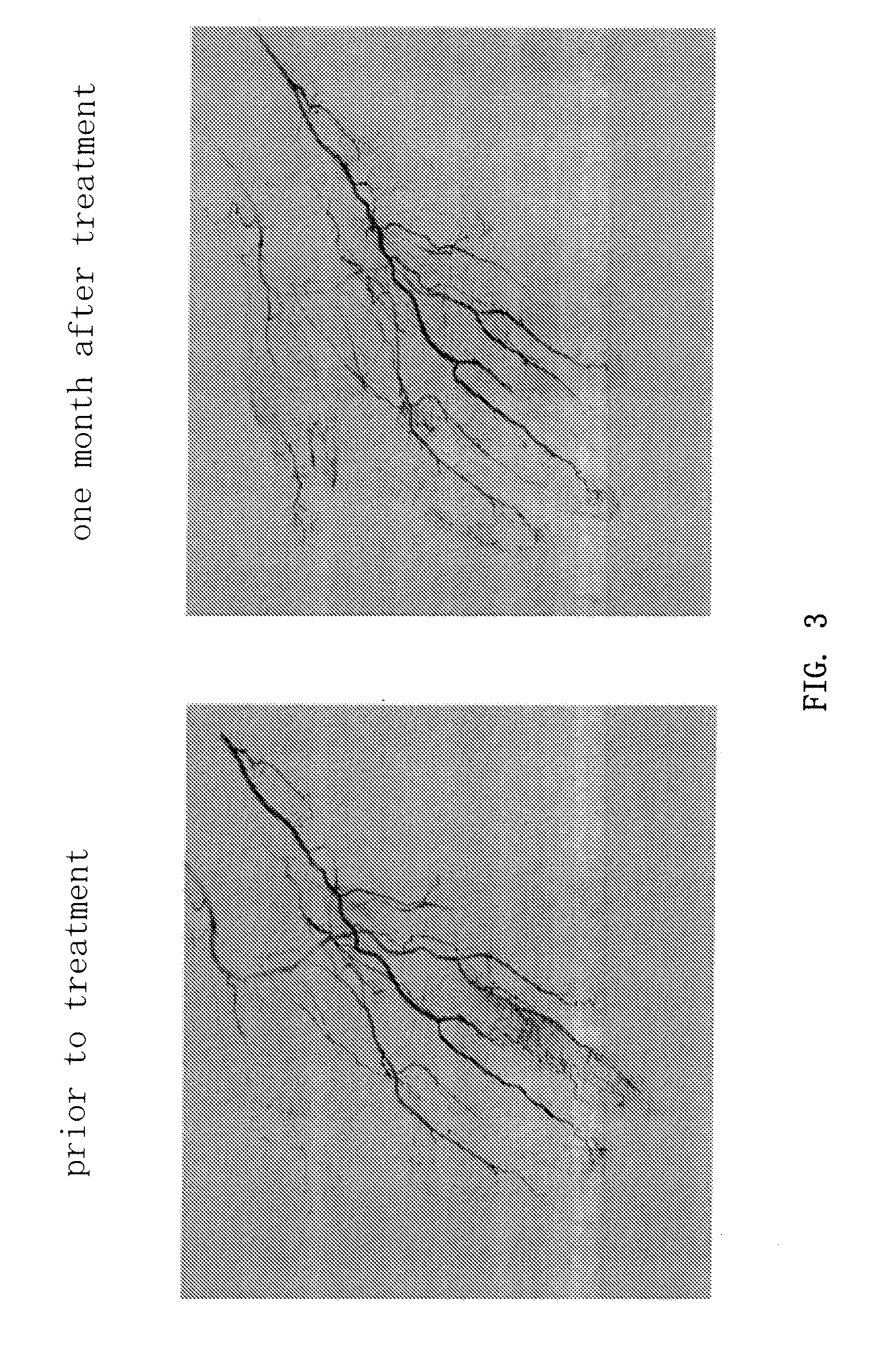
Disclosed is a method for stimulating revascularization in a subject comprising the steps of:(a) administering erythropoietin to the subject;(b) collecting peripheral blood mononuclear cells from the subject; and(c) administering the collected peripheral blood mononuclear cells to a target site of the subject. Peripheral blood mononuclear cells, and particularly CD34-positive cells, are mobilized into the peripheral blood of a subject by the administration of erythropoietin to the subject. The method of the present invention is useful for the treatment of ischemic diseases, such as peripheral vascular disorder.
Owner:CHUGAI PHARMA CO LTD +1
Stem cell mediated neuroregeneration and neuroprotection
InactiveUS20170258843A1Reduce redundancyReduced ability to evokeMammal material medical ingredientsProgenitorNervous system
Disclosed are means of inducing neuroregeneration and / or neuroprotection in patients with damage to the nervous system. In one embodiment, placenta derived CD34 positive cells are administered to a patient suffering from a neurological injury, said cells administered alone, or in combination with endothelial progenitor cells that are derived from placental sources. In one embodiment cells are manipulated to decrease immunogenicity by means of gene-editing or RNA interference inducing means. In another embodiment, neuroprotection and / or neuroregeneration is achieved by administration of exosomes derived from placental stem cells.
Owner:ANGIOSTEM INC
Method for Inducing Differentiation of Myeloid-Derived Suppressor Cells from Cord - Blood CD34 Positive Cells and Proliferating Same, and use of Myeloid-Derived
InactiveUS20180216065A1Mammal material medical ingredientsSkeletal/connective tissue cellsCord blood stem cellMyeloid-derived Suppressor Cell
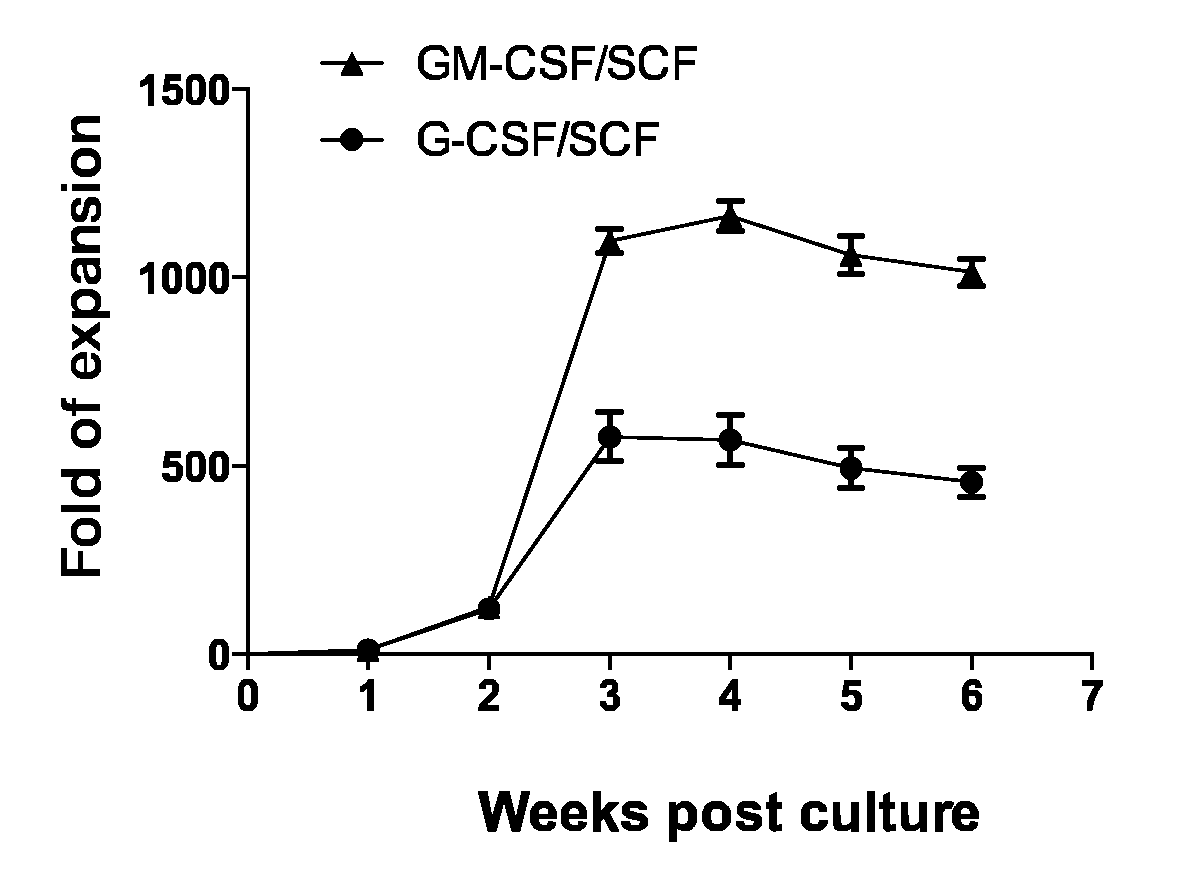
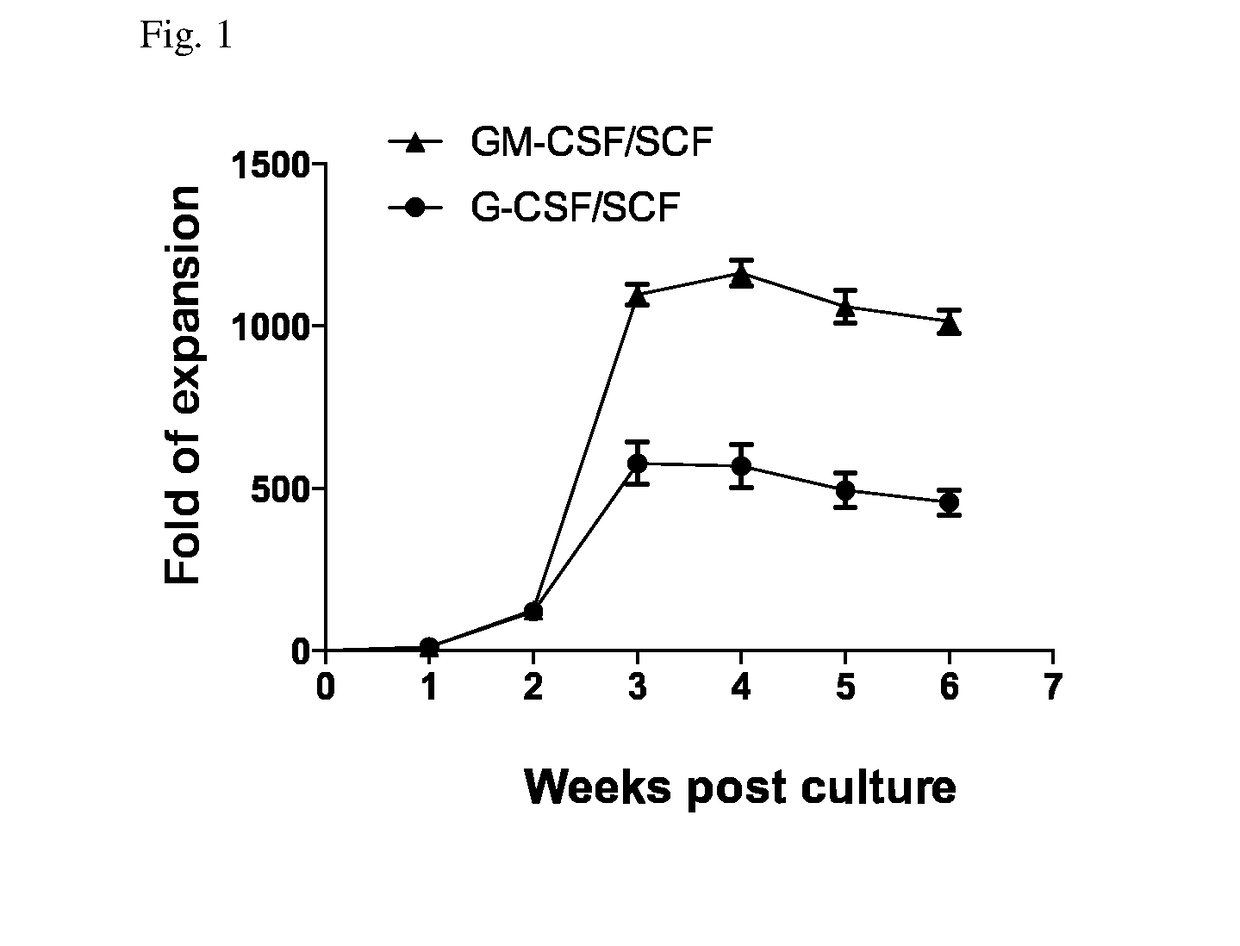
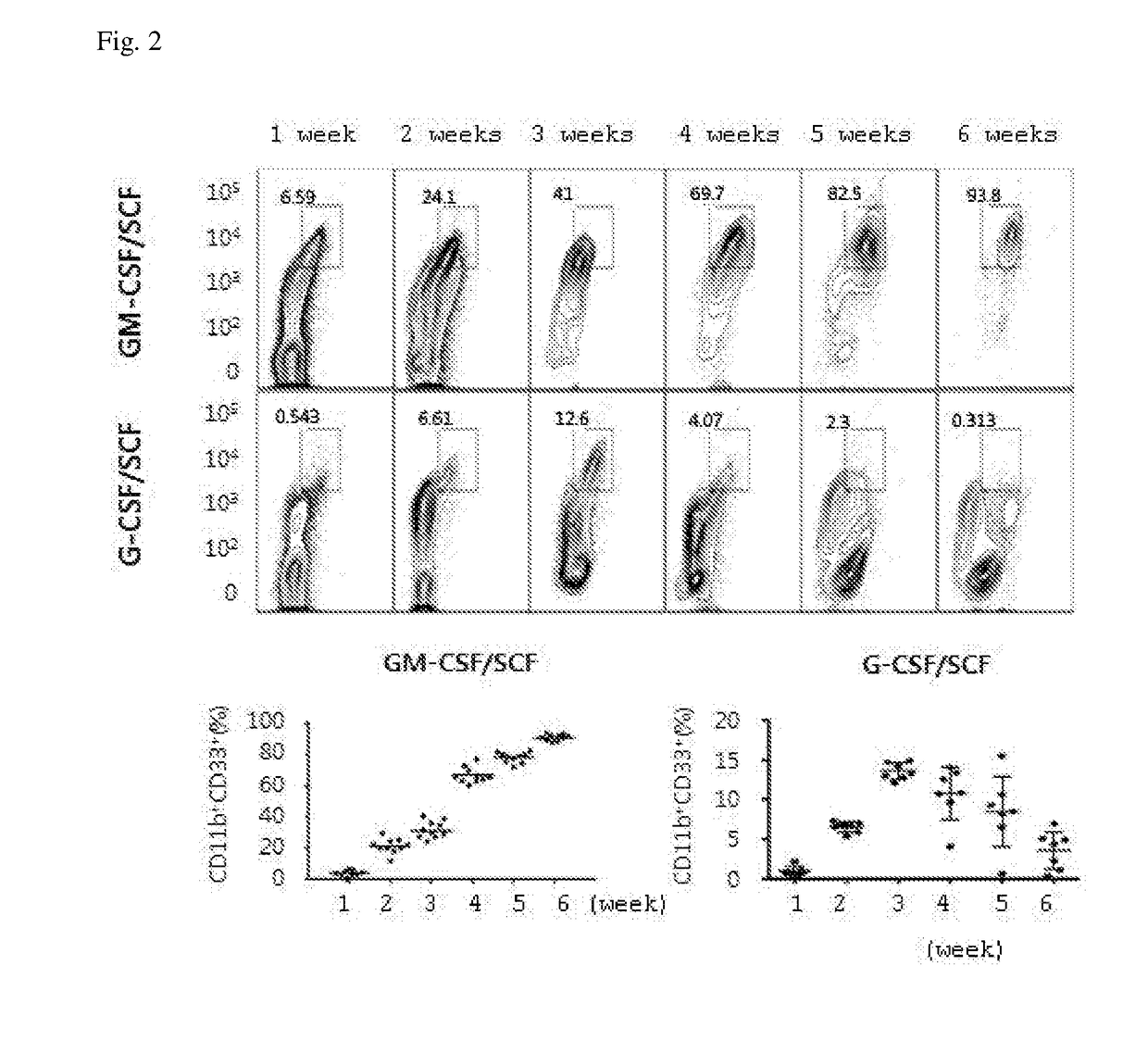
The present invention relates to a method for inducing differentiation myeloid-derived suppressor cells from cord blood CD34 positive cells and proliferating the same, and a use of the myeloid-derived suppressor cells. More specifically, myeloid-derived suppressor cells are induced to differentiate and proliferated by culturing cord blood CD34 positive cells in the presence of a cytokine cocktail of GM-CSF and SCF, such that myeloid-derived suppressor cells can be mass-produced in vitro, and the myeloid-derived suppressor cells can be used in preventing or treating immunorejection-related diseases such as graft-versus-host disease.
Owner:THE CATHOLIC UNIV OF KOREA IND ACADEMIC COOP FOUND
Method for separating hematopoietic stem cells from umbilical cord blood and amplifying CD34 positive cells
InactiveCN106701682AGood differentiation effectEasy to operateCulture processMammal material medical ingredientsFicollBiology
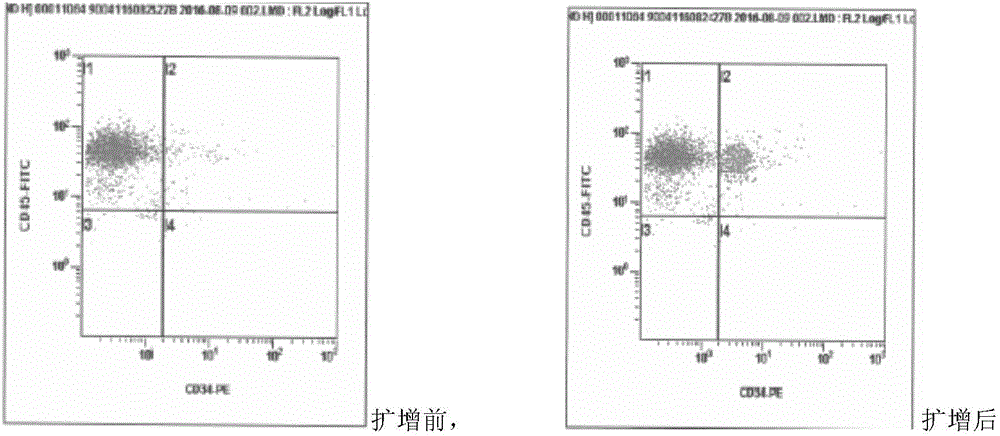
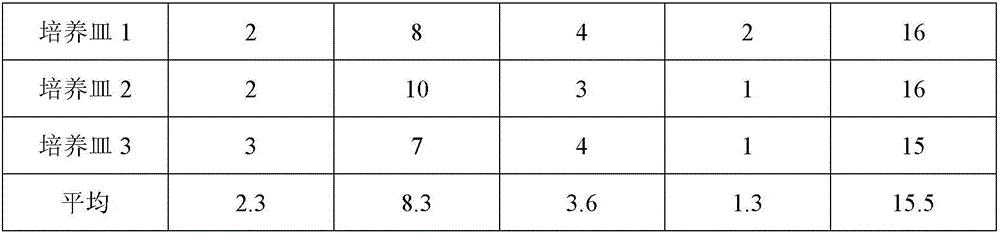
The invention relates to a method for separating hematopoietic stem cells from umbilical cord blood and amplifying CD34 positive cells. The method comprises the following specific steps: separating hematopoietic stem cells from umbilical cord blood; purifying; separating by using a Ficoll separating solution; counting the CD34 positive cells by using a flow cytometer, diluting, inoculating and culturing; changing the solution and culturing; transferring the cells to new plates and culturing; evaluating the cell amplifying effect. The invention further relates to cell products containing the CD34 positive cells prepared by the method and application of the cell products. The method has excellent technical effects as described in the specification.
Owner:BOYALIFE
Preparation method of placental hematopoietic stem cells
ActiveCN109337871ANo damageHelp dissociationCell dissociation methodsDead animal preservationCell activityCryopreservation
The invention belongs to the field of biotechnology, and particularly relates to a preparation method of placental hematopoietic stem cells. The preparation method comprises the following steps: (1) pretreatment of a placenta; (2) first lavage of the placenta; (3) second lavage of the placenta; (4) third lavage of the placenta; (5) mixing and centrifuging; (6) primary purification of the placenta;and (7) cryopreservation of hematopoietic stem cells. The preparation method can improve the separation quantity and cell activity of CD34 positive cells, the adopted reagents have no potential risks, and can be applied clinically, and the experimental cost is reduced.
Owner:山东康华生物医疗科技股份有限公司
Method for increasing extraction rate of CD34 positive stromal vascular fraction (SVF) in high-fat tissue
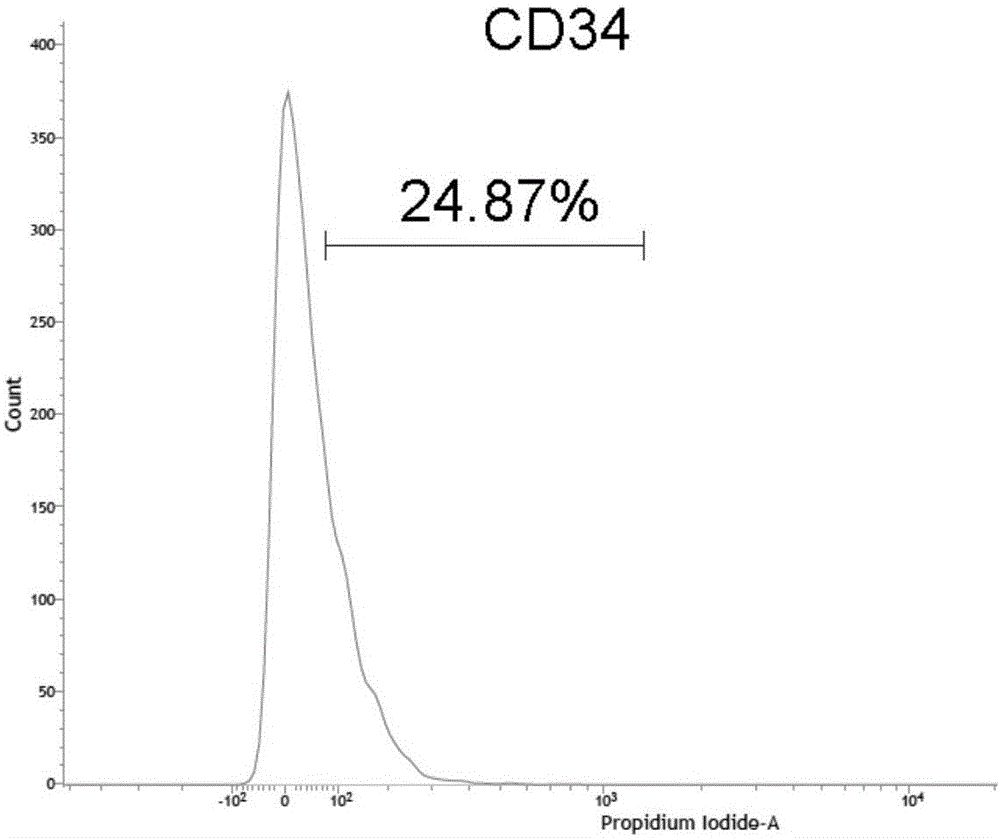
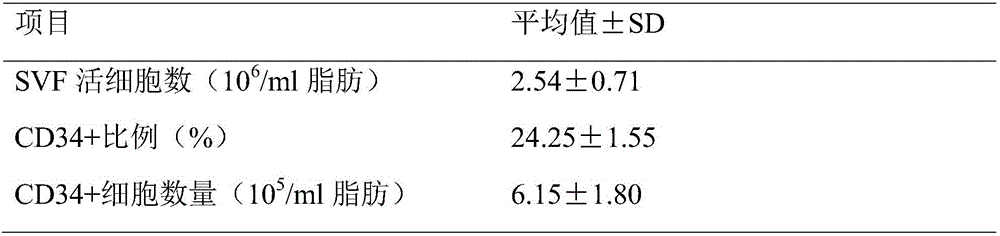
The invention discloses a method for increasing the extraction rate of the CD34 positive stromal vascular fraction (SVF) in high-fat tissue. The method includes the steps that fat tissue is subjected to suspension with normal saline, centrifugation is carried out to remove an oil layer, sediment is repeatedly centrifuged, sediment obtained in the last time of centrifugation is crushed, and crushed tissue is obtained; then, a collagenase aqueous solution is added into the crushed tissue, oscillation is carried out at 37 DEG C for digestion, centrifugation is carried out after digestion is finished, and sediment, namely, the CD34 positive SVF is taken. By means of the method, low-concentration collagenase can be used for digestion, and the SVF containing high-ratio CD34 positive stem / progenitor cells is obtained from the fat tissue; the ratio of CD34 positive stem / progenitor cells in the SVF is larger than 24%, and more than 6.0*10<5> CD34 positive cells can be obtained from each milliliter of fat tissue.
Owner:HANGZHOU S EVANS BIOSCI LTD
Method of detecting leukemic cell
InactiveUS20110014636A1Conveniently obtainMicrobiological testing/measurementBiological material analysisBlood specimenSeverity/Intensity
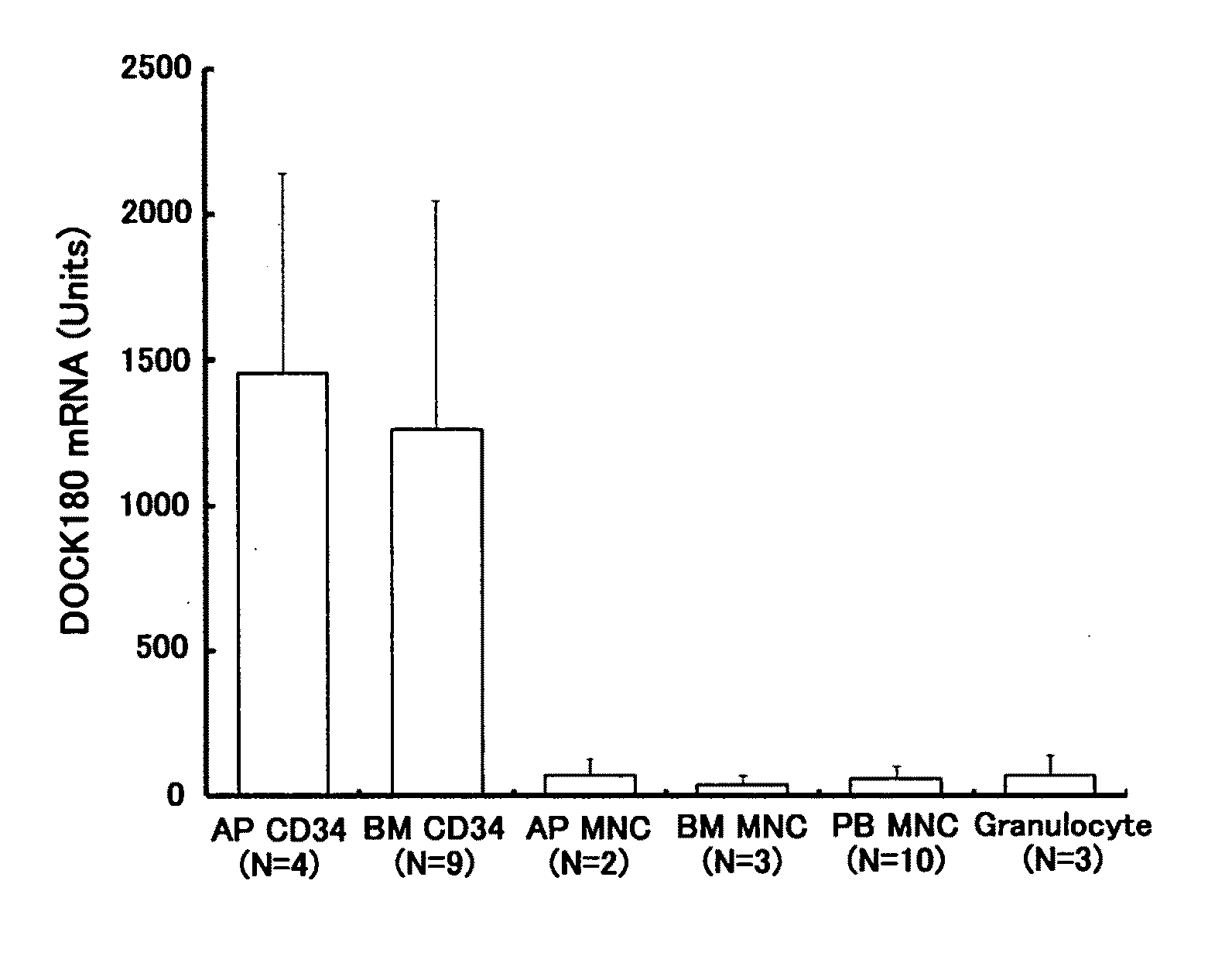
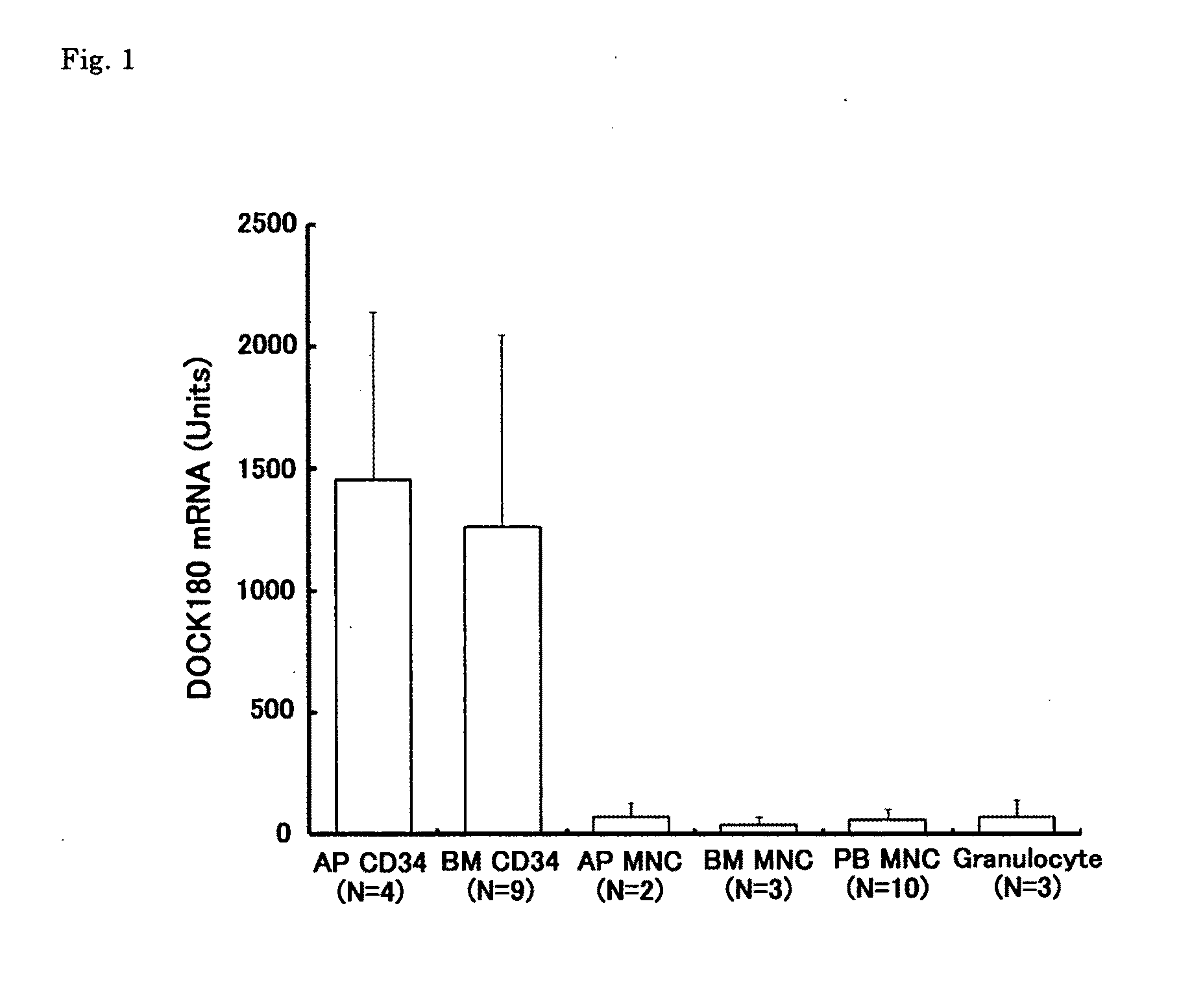
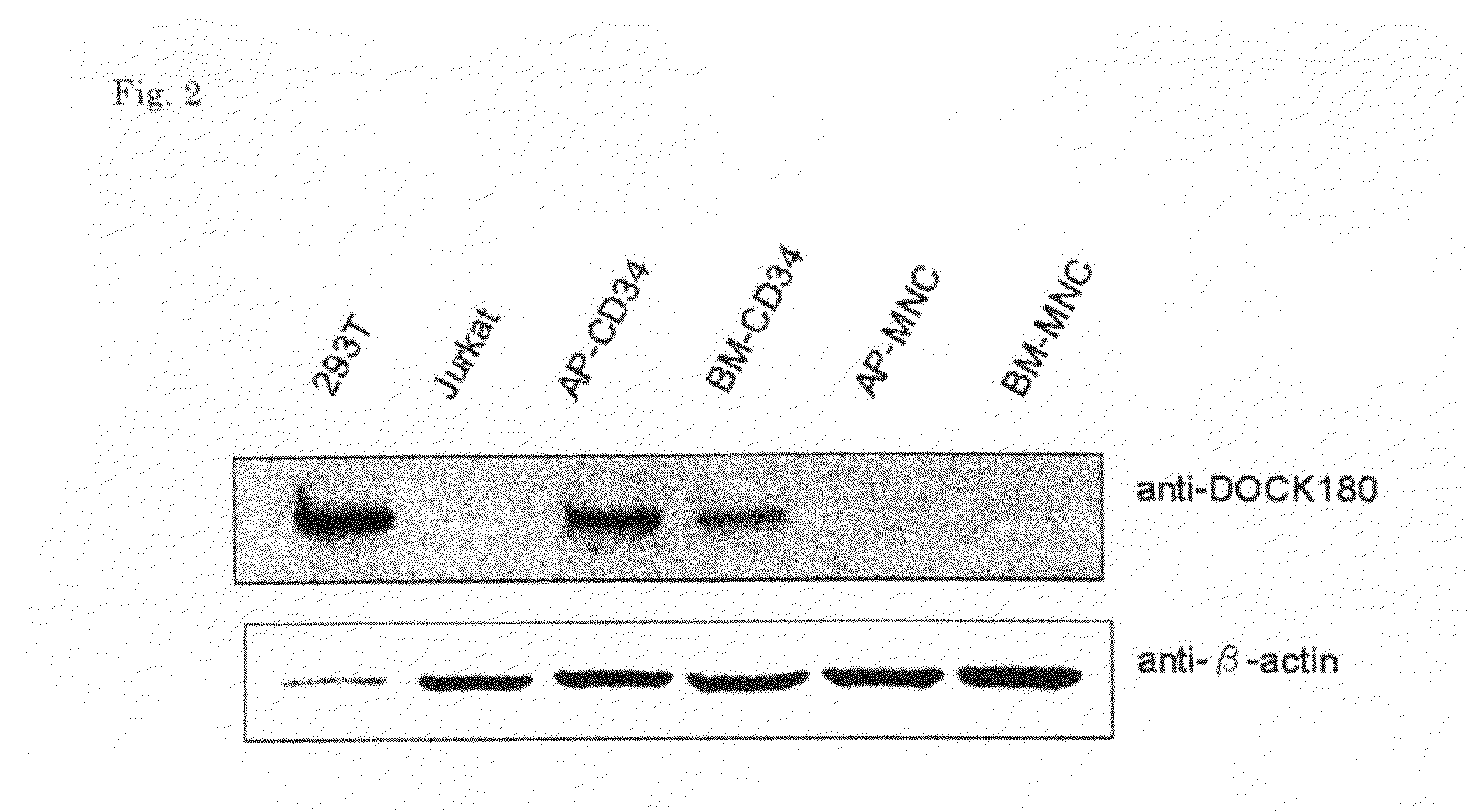
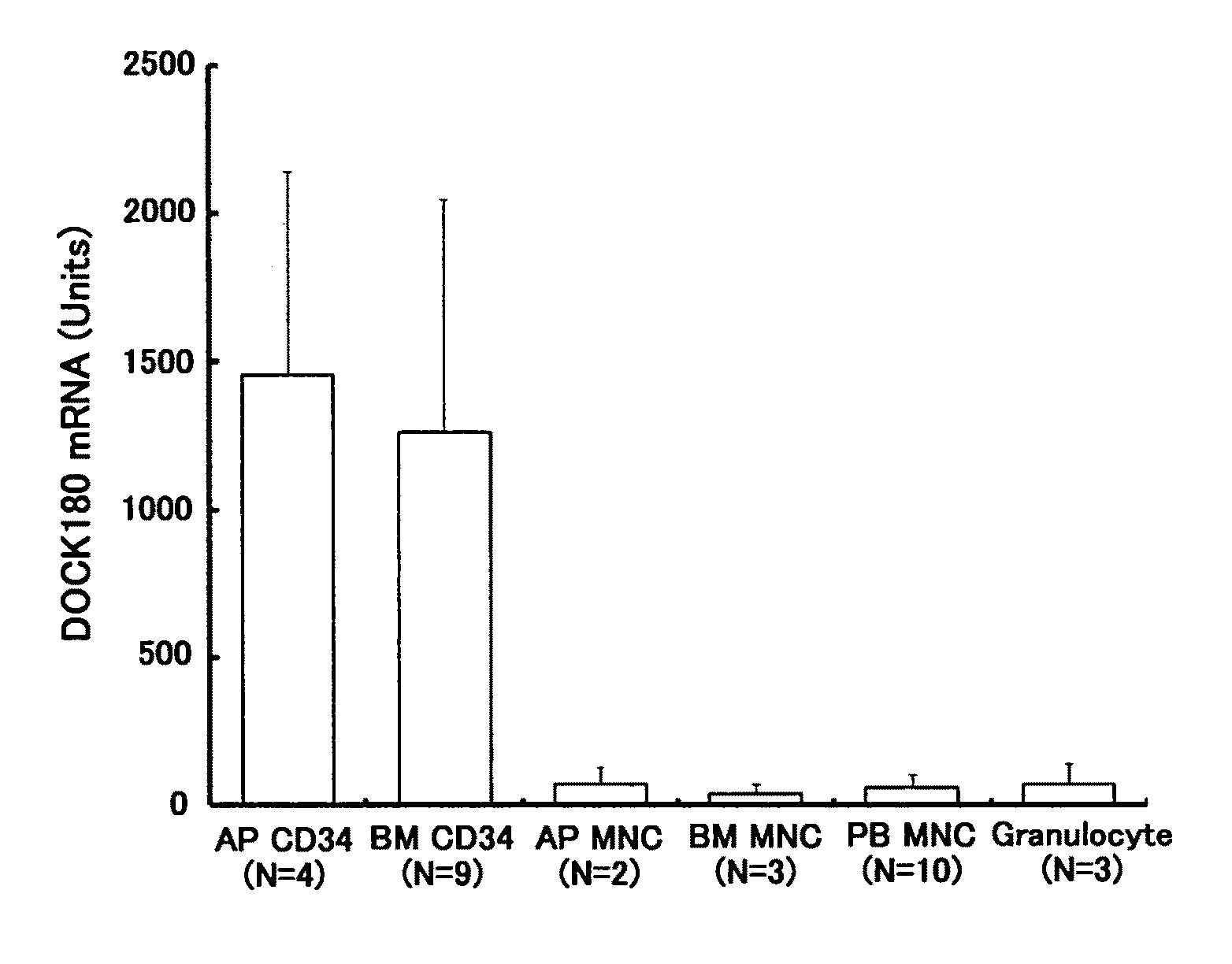
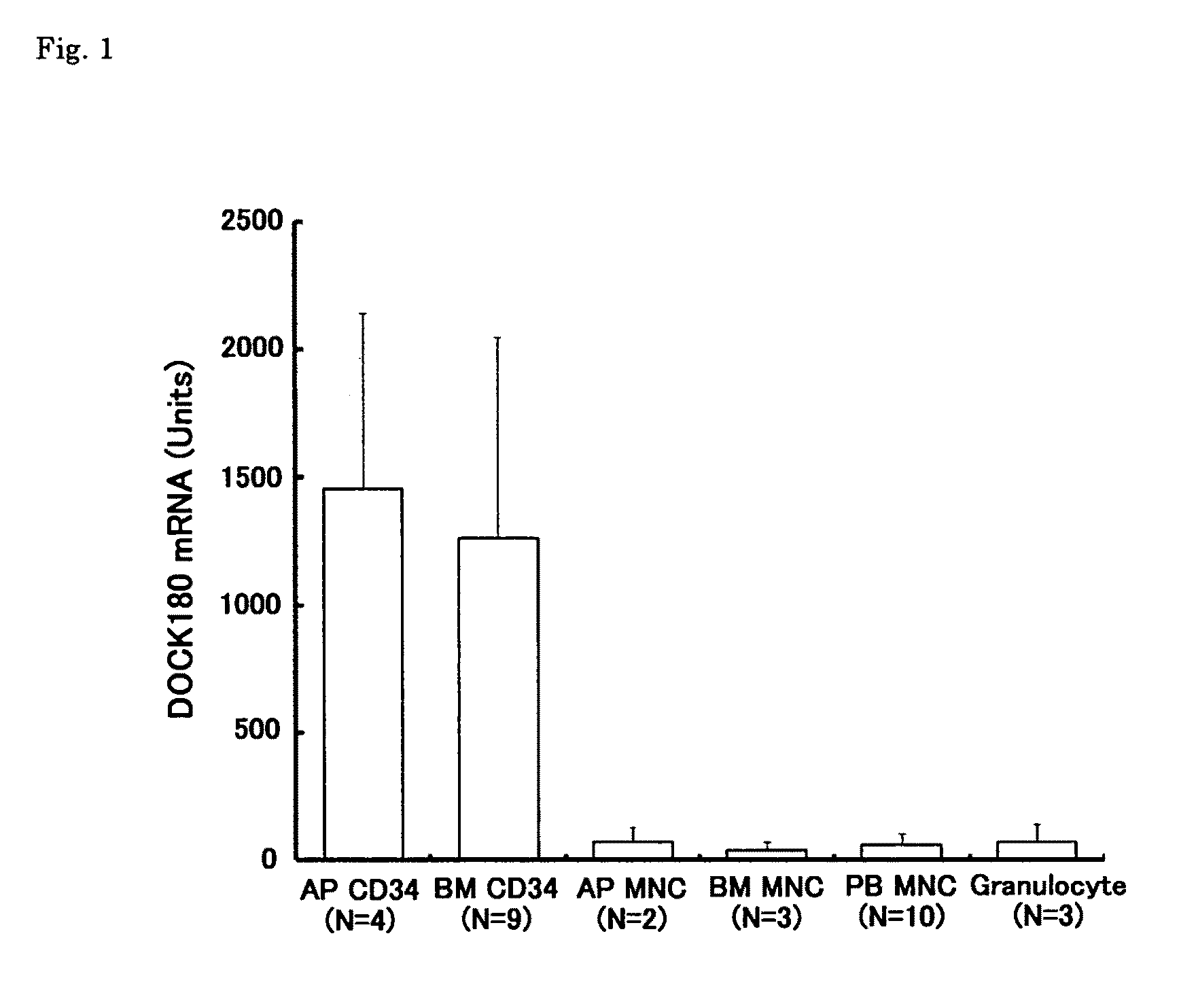
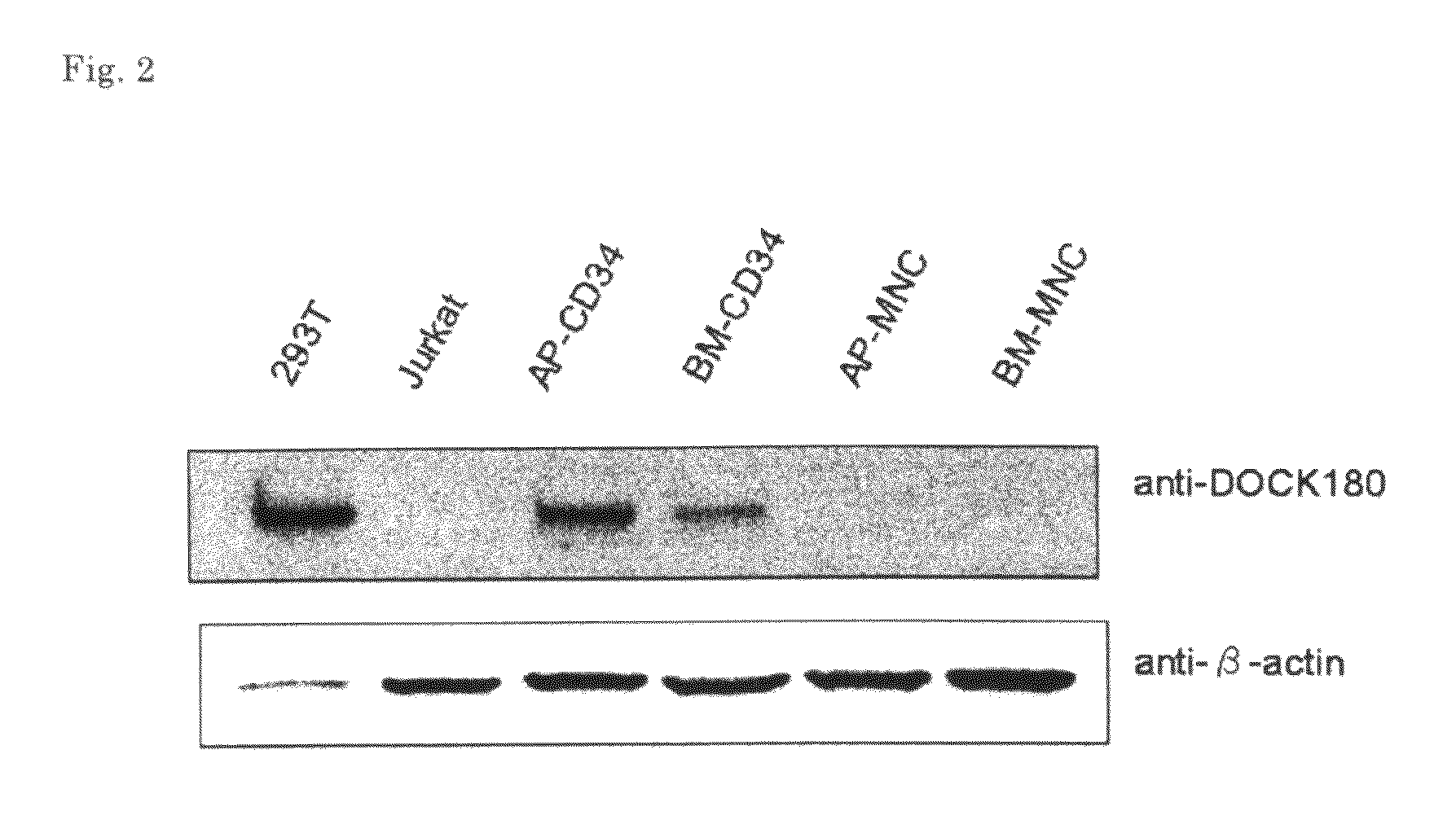
[Problems] To provide a marker which is useful in diagnosing leukemia and a method of using the same.[Means for Solving Problems] A method of detecting a leukemia cell which comprises detecting a CD34-positive cell being negative in DOCK180 expression from a blood specimen. According to this method, a DOCK180 expression pattern specific to a tumor cell in leukemia, in particular, acute leukemia is confirmed so that information which is useful in diagnosing leukemia and monitoring and determining the severity and degree of recovery of the same by a doctor can be obtained by a convenient procedure using blood specimens and thus provided.
Owner:HOKKAIDO UNIVERSITY
ASD (autism spectrum disorder) detection kit
InactiveCN107674913AEffective preventionEffective therapeuticMicrobiological testing/measurementIntervention treatmentAutism spectrum disorder
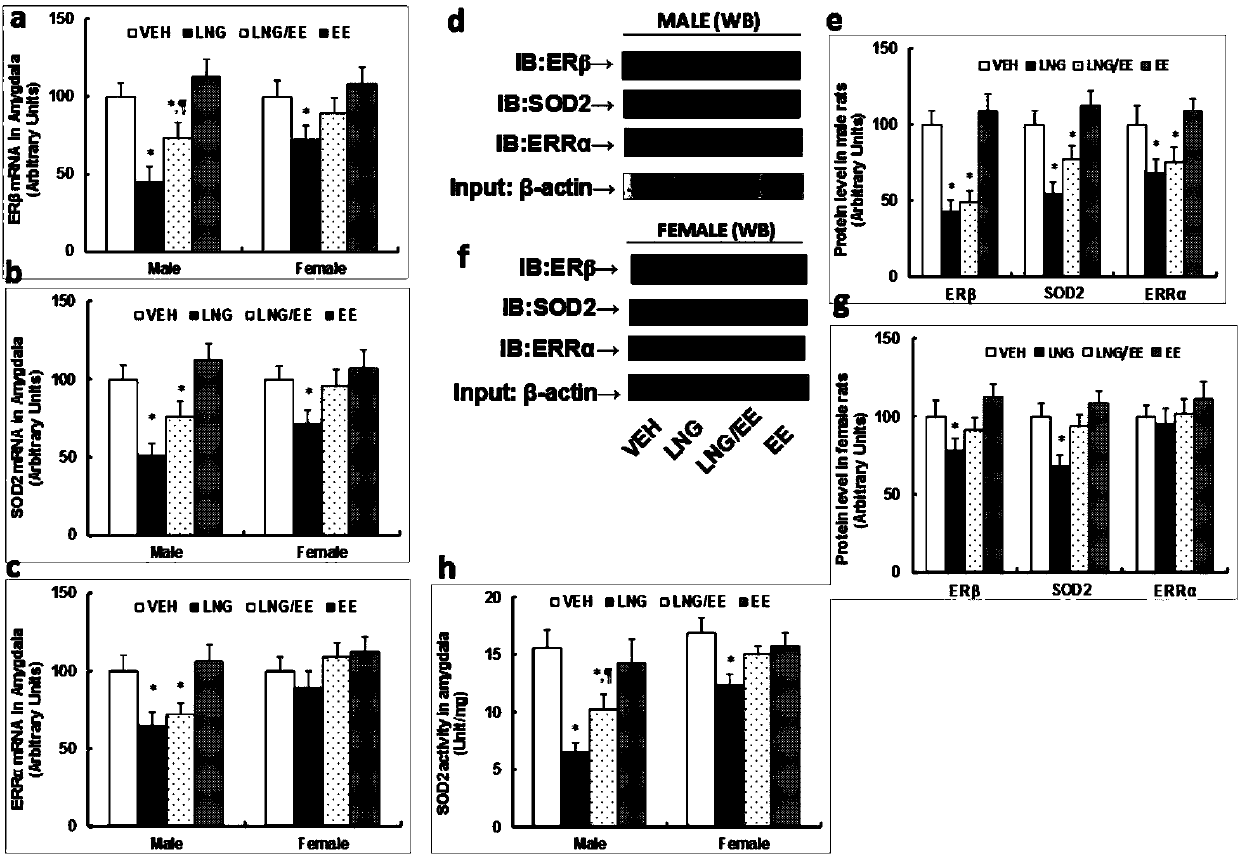
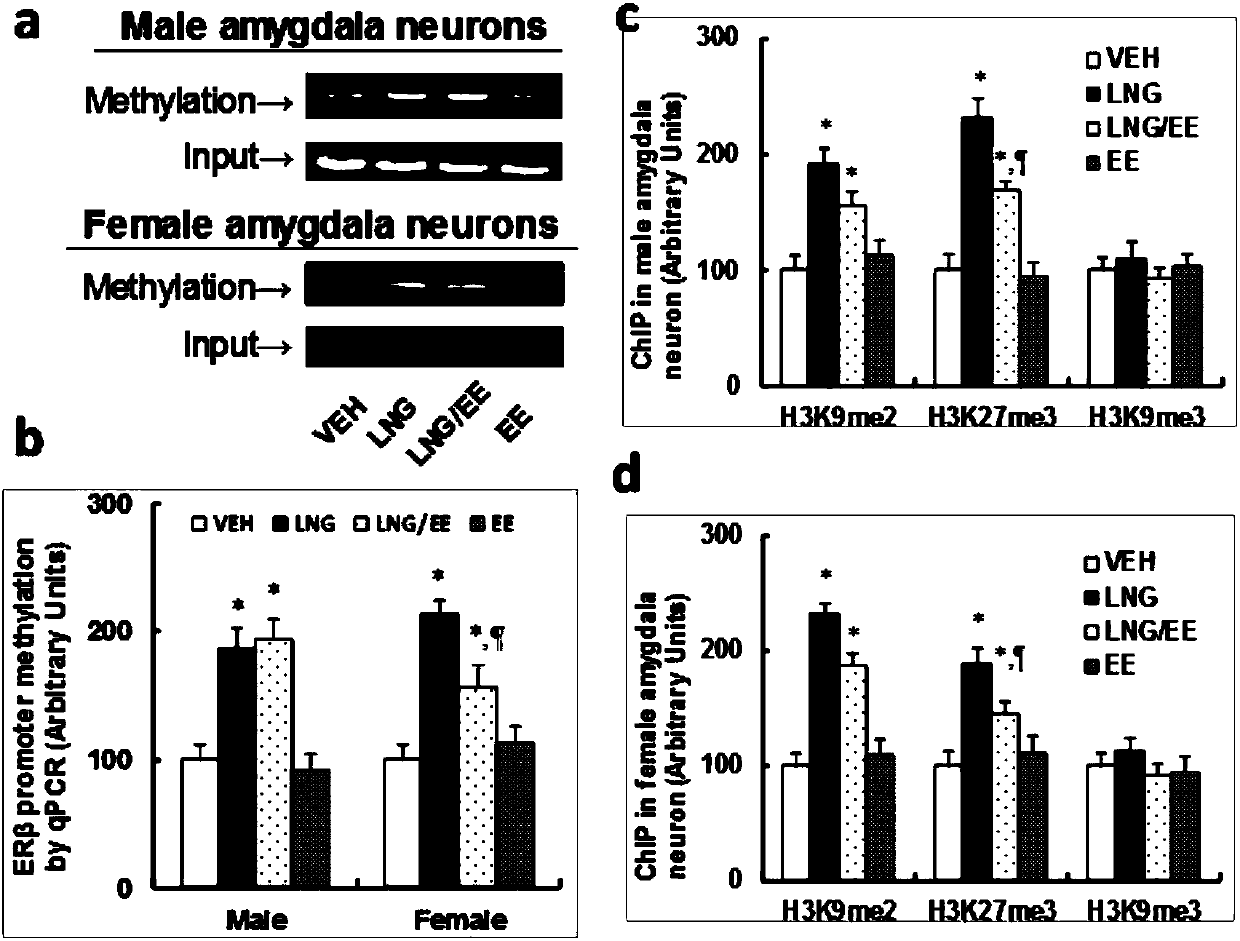
The invention belongs to the technical field of biology and discloses an ASD (autism spectrum disorder) detection kit. Discoveries find that expression of CD34 positive cell ER beta in bone marrow andperipheral blood and related genes of the CD34 positive cell ER beta is closely related with production of ASD-like behaviors and can serve as markers of ASD for detection and pre-screening of ASD. The ASD detection kit can be used for clinically detecting and pre-screening ASD rapidly, sensitively and specifically, and effective preventing and intervention treatment measures can be taken for children patients with ASD according to detection results.
Owner:GUANGZHOU SALIAI STEMCELL SCI & TECH CO LTD
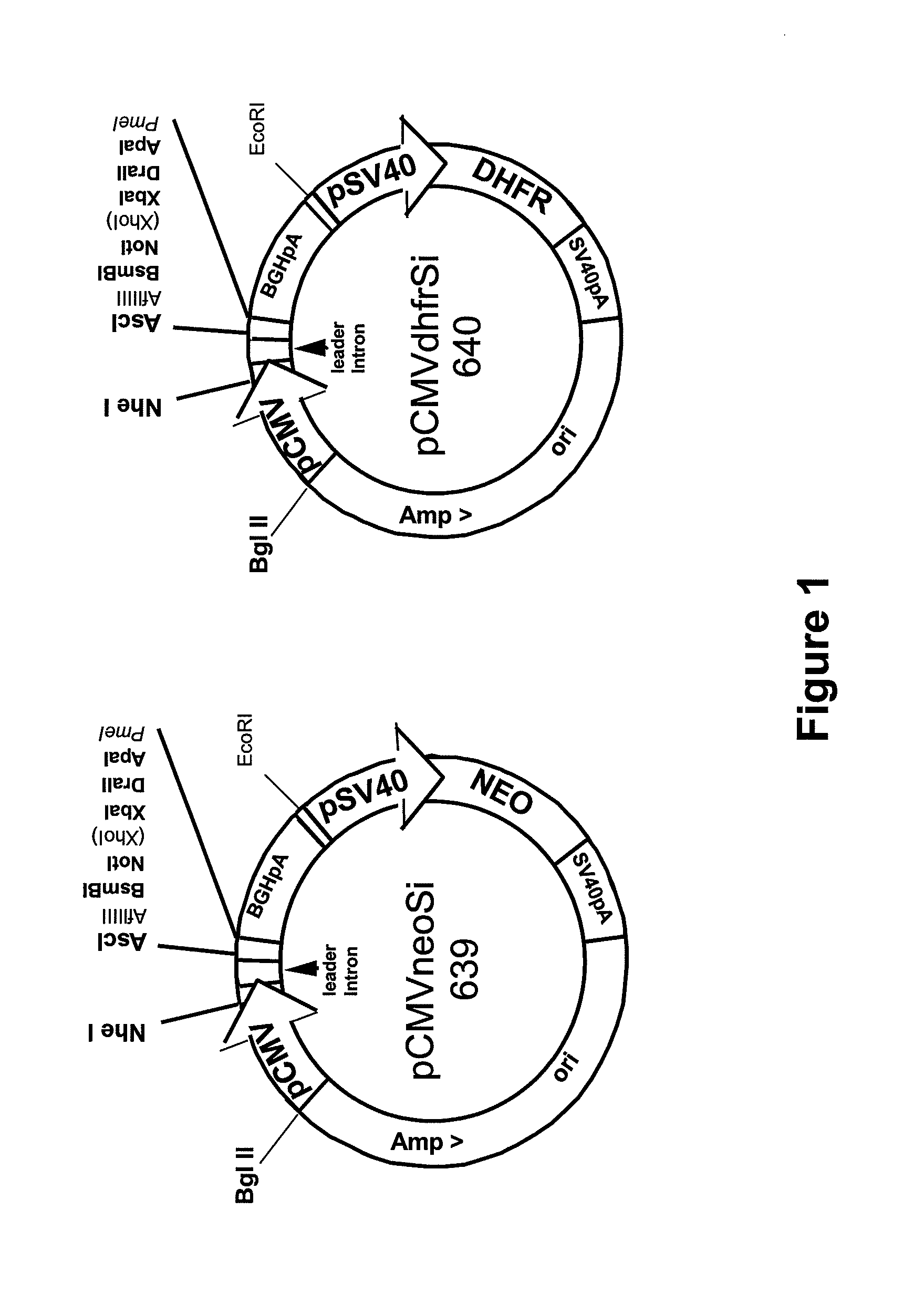
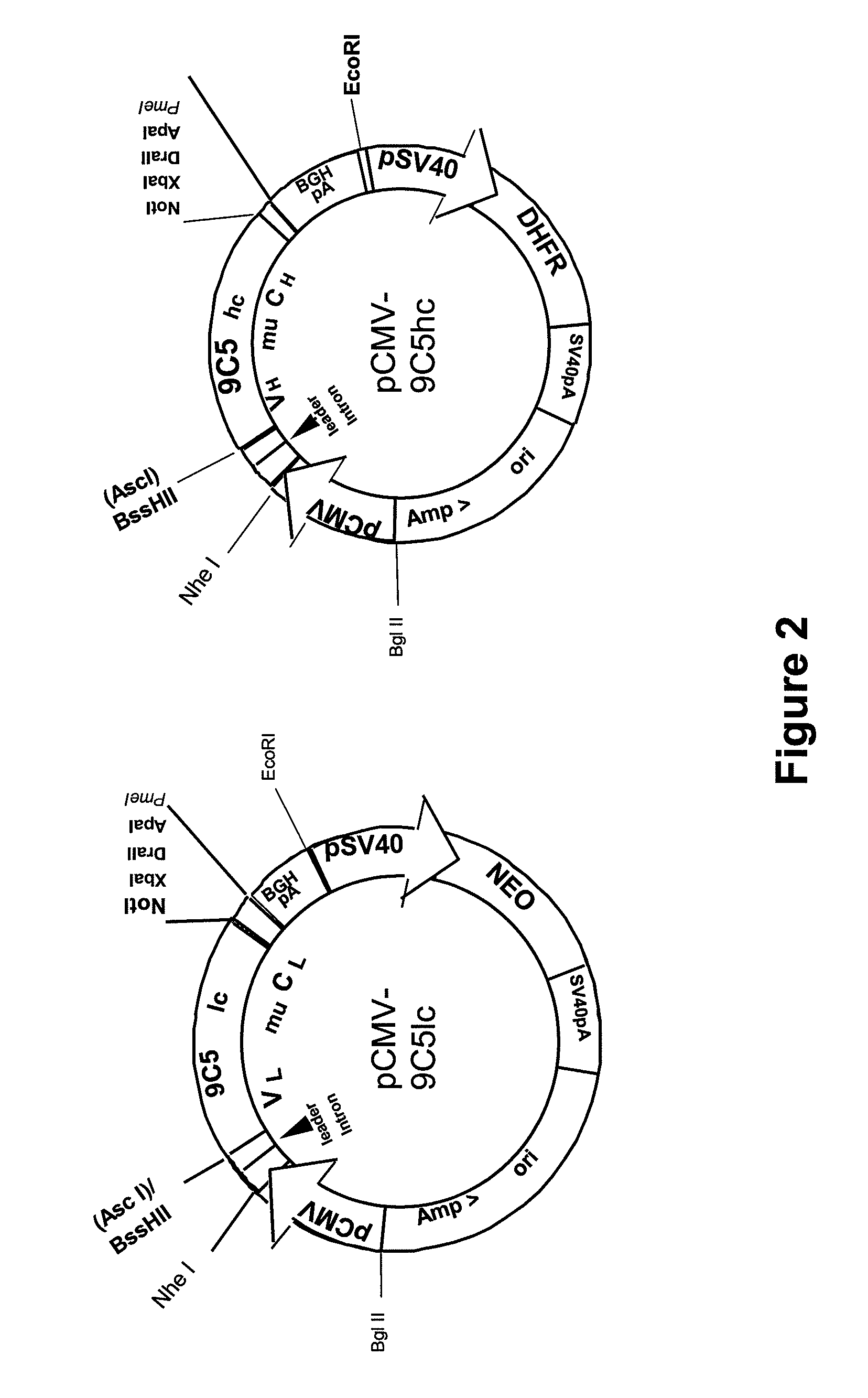
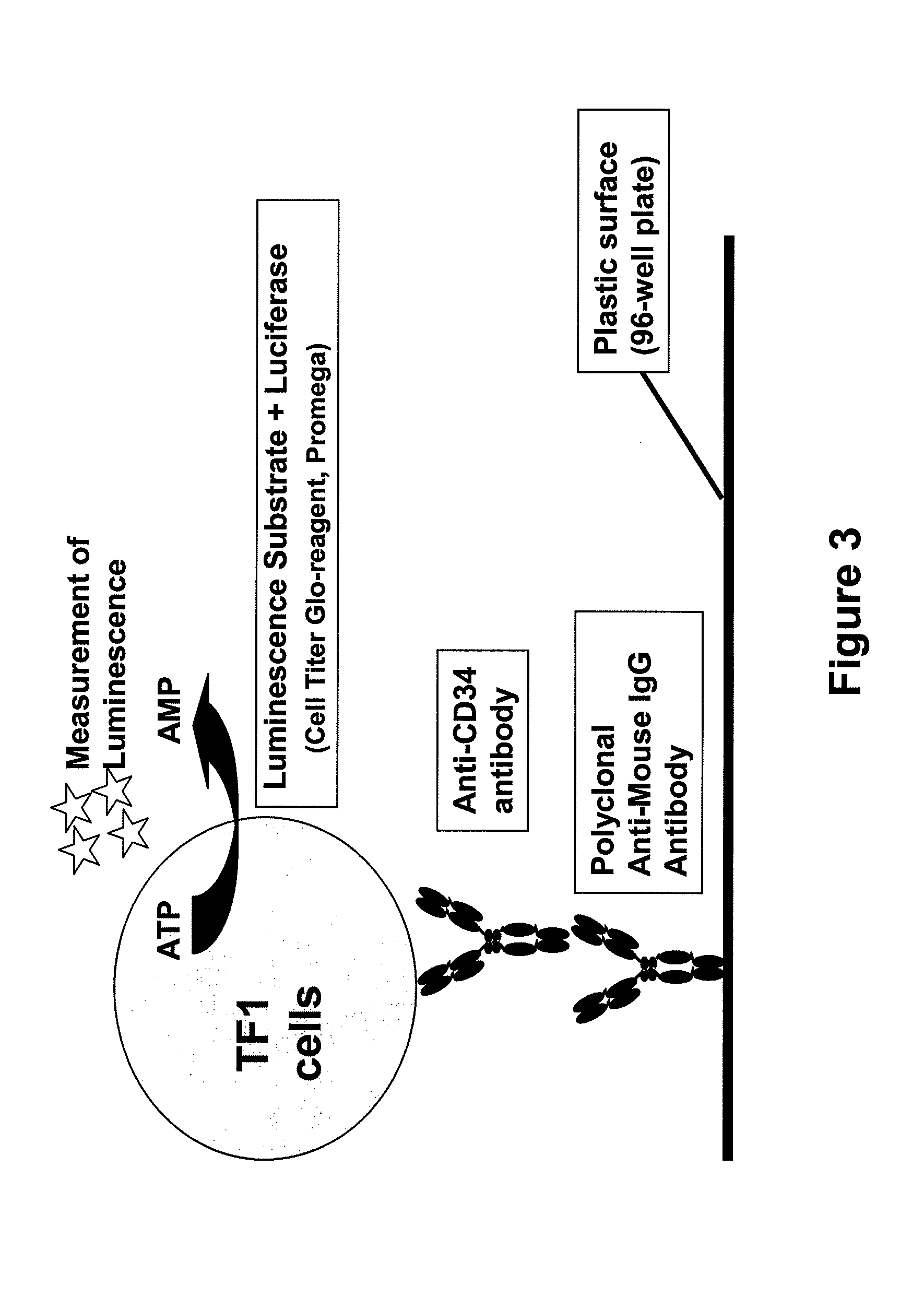
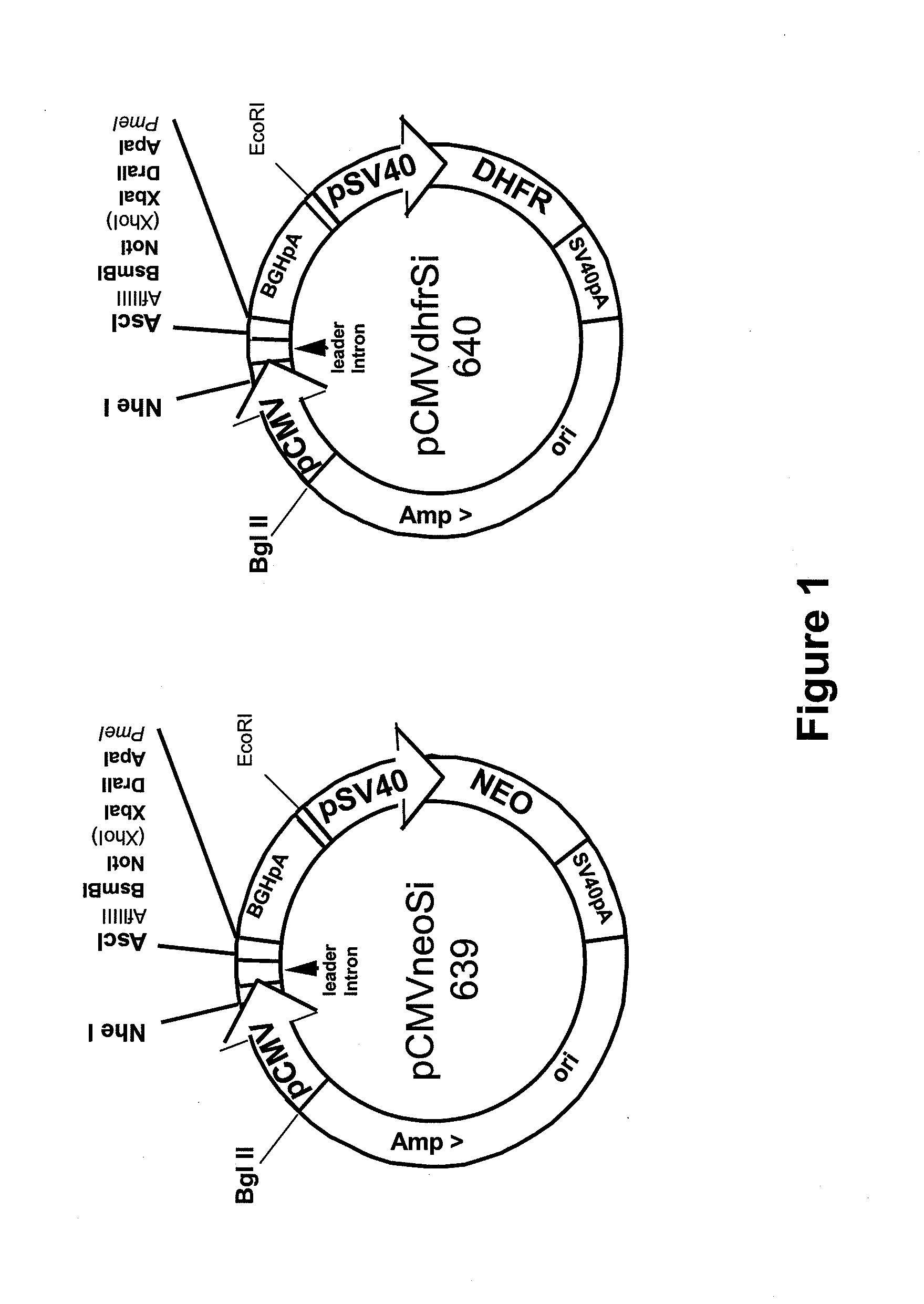
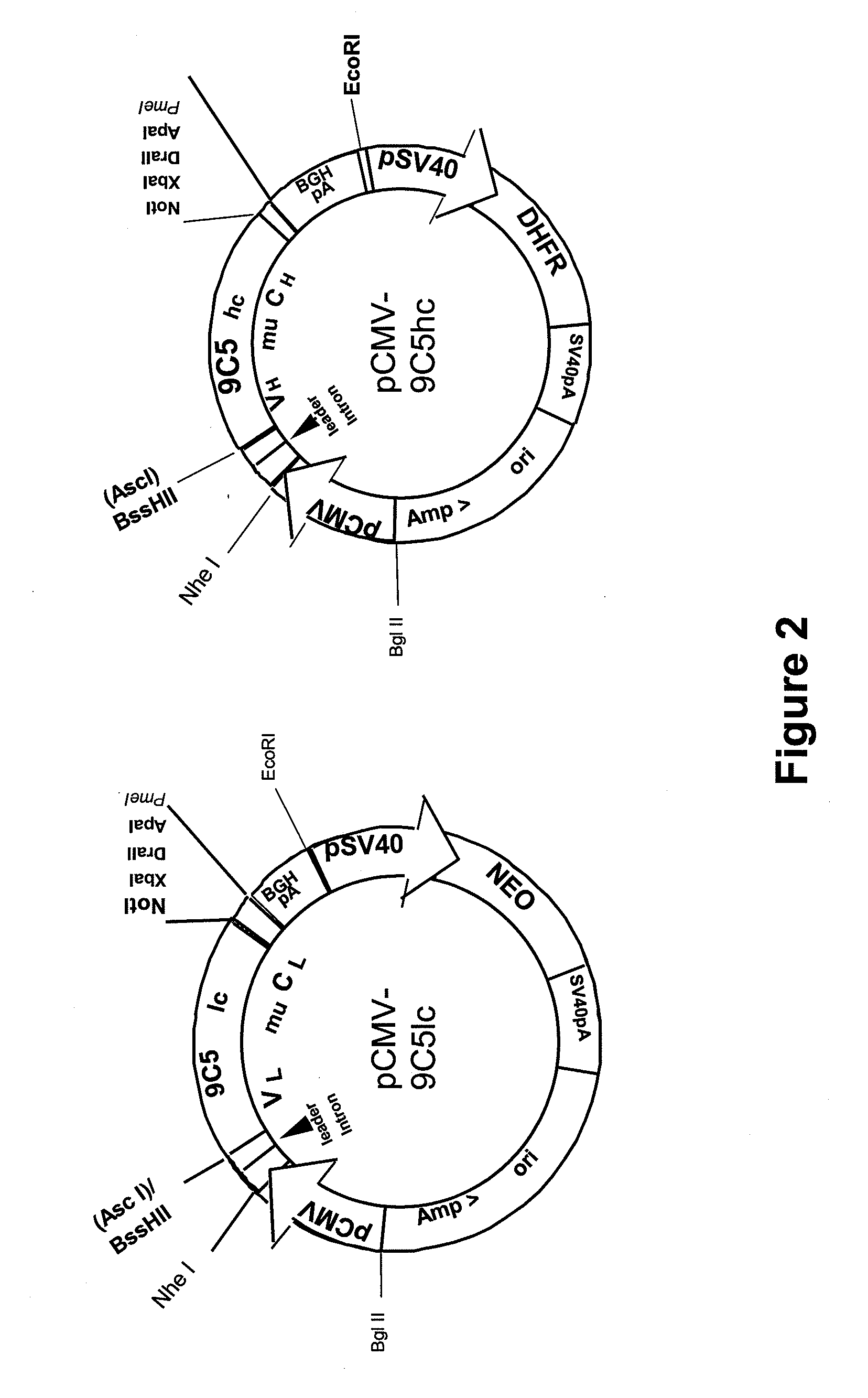
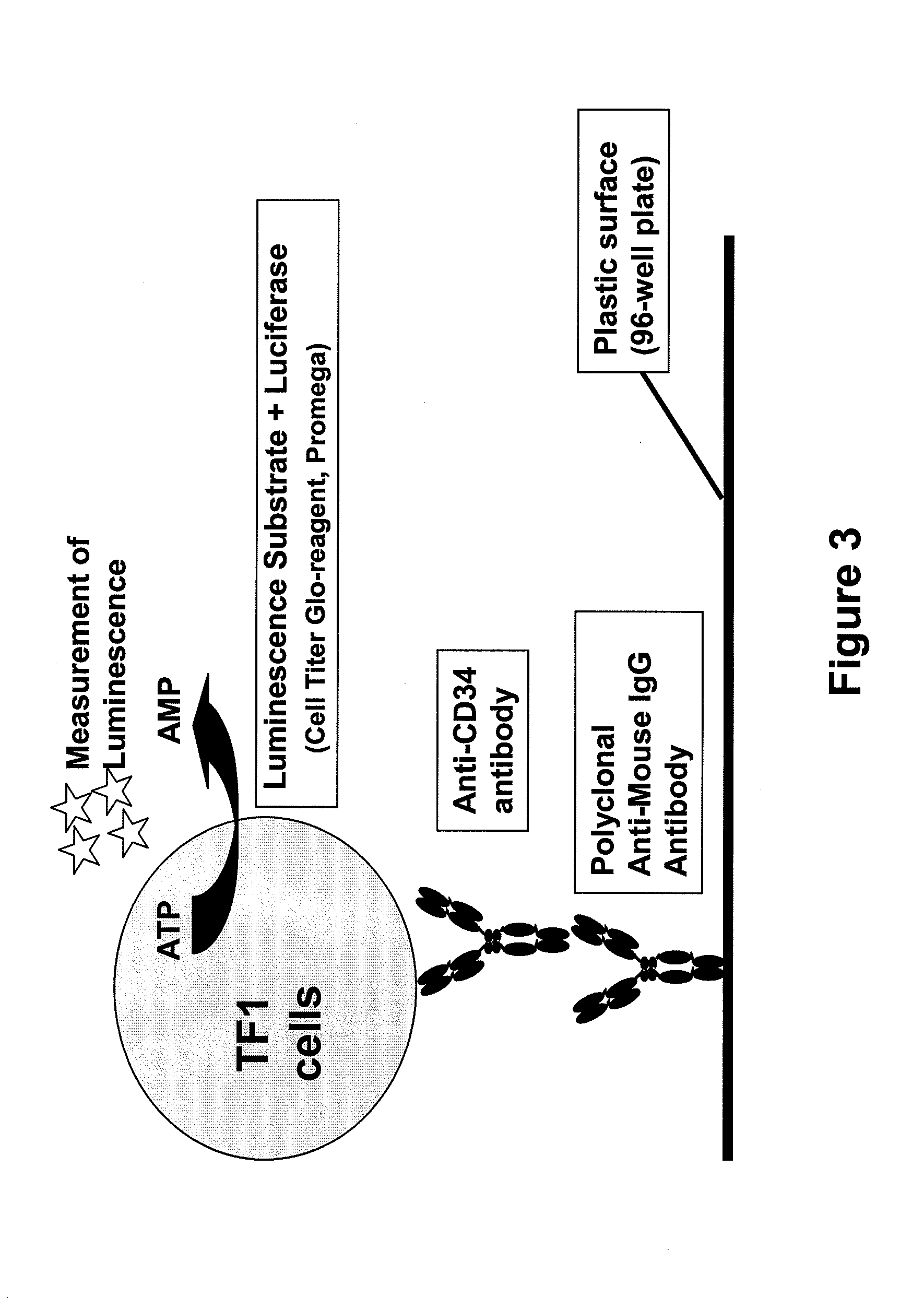
Procedure for the generation of a high producer cell line for the expression of a recombinant anti-CD34 antibody
The present invention relates to cell capture assay for the selection of a high producer cell line expressing anti-CD34 antibodies that recognize the CD34 membrane-protein in the cell membrane. The monoclonal antibody secreted by the hybridoma cell line 9C5 / 9069 binds to human CD34 and is used to isolate stem cells. The DNA sequences encoding for the antibody heavy and light chain have been identified, isolated from the hybridoma cells and cloned into appropriate expression vectors. After co-transfection of the heavy and light chain genes into HEK293T or in CHO cells either conditioned medium or purified antibody were assessed for binding to CD34 protein located in the cell membrane in different cell capture assays. The binding of the antibody to CD34-positive cells could be shown with these assays for several cell lines.
Owner:TAKEDA PHARMA CO LTD
Method of screening endothelial cells for angiogenic capability
InactiveUS7547518B2Material analysis by observing effect on chemical indicatorMicrobiological testing/measurementAngiogenesis growth factorBlood vessel
Provided is a method of screening a primary endothelial cell population for angiogenesis capability comprising: (a) measuring the percentage of cells that are positive for VEGF R2 and CD34, the level of VEGF R2, or measuring the VEGF R2 to VEGF R1 ratio in the population; and (b) selecting those populations where the measured percentage or the measured ratio is over a threshold value.
Owner:CORNING INC
Procedure for the generation of a high producer cell line for the expression of a recombinant Anti-CD34 antibody
ActiveUS20090221003A1Animal cellsImmunoglobulins against cell receptors/antigens/surface-determinantsCell membraneCell Membrane Proteins
The present invention relates to cell capture assay for the selection of a high producer cell line expressing anti-CD34 antibodies that recognize the CD34 membrane-protein in the cell membrane. The monoclonal antibody secreted by the hybridoma cell line 9C5 / 9069 binds to human CD34 and is used to isolate stem cells. The DNA sequences encoding for the antibody heavy and light chain have been identified, isolated from the hybridoma cells and cloned into appropriate expression vectors. After co-transfection of the heavy and light chain genes into HEK293T or in CHO cells either conditioned medium or purified antibody were assessed for binding to CD34 protein located in the cell membrane in different cell capture assays. The binding of the antibody to CD34-positive cells could be shown with these assays for several cell lines.
Owner:TAKEDA PHARMA CO LTD
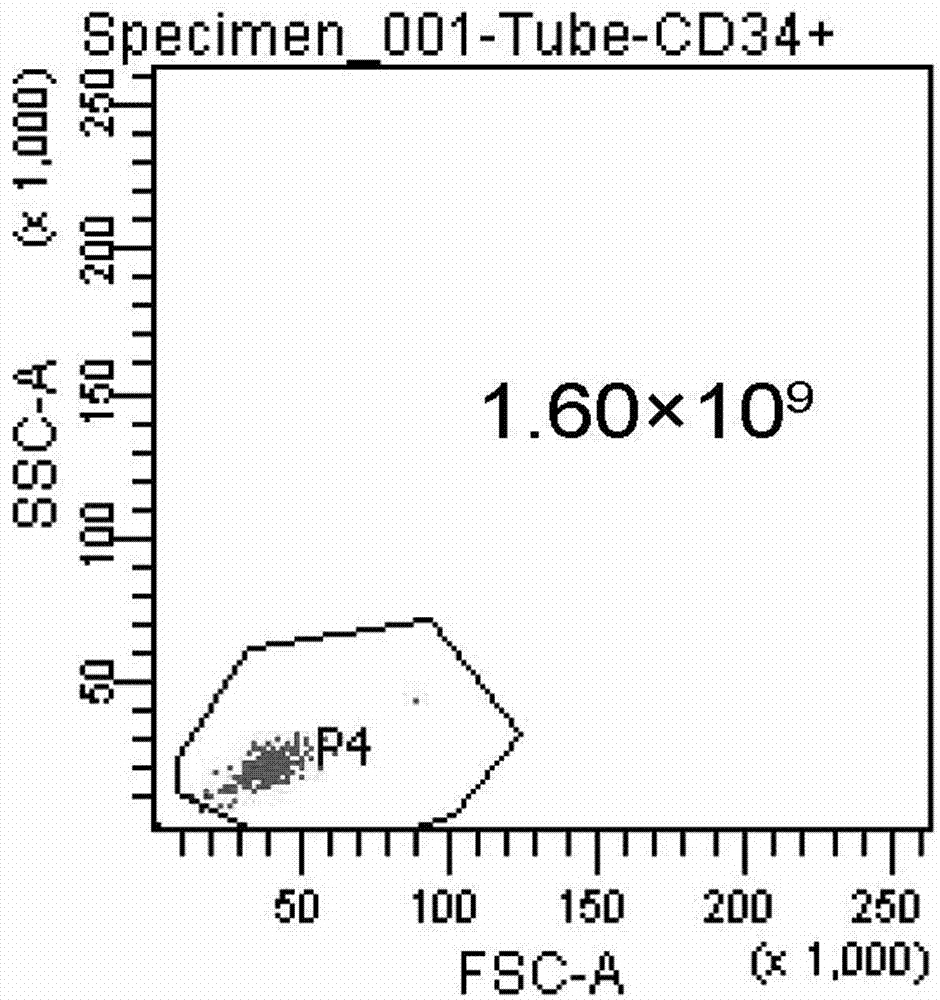
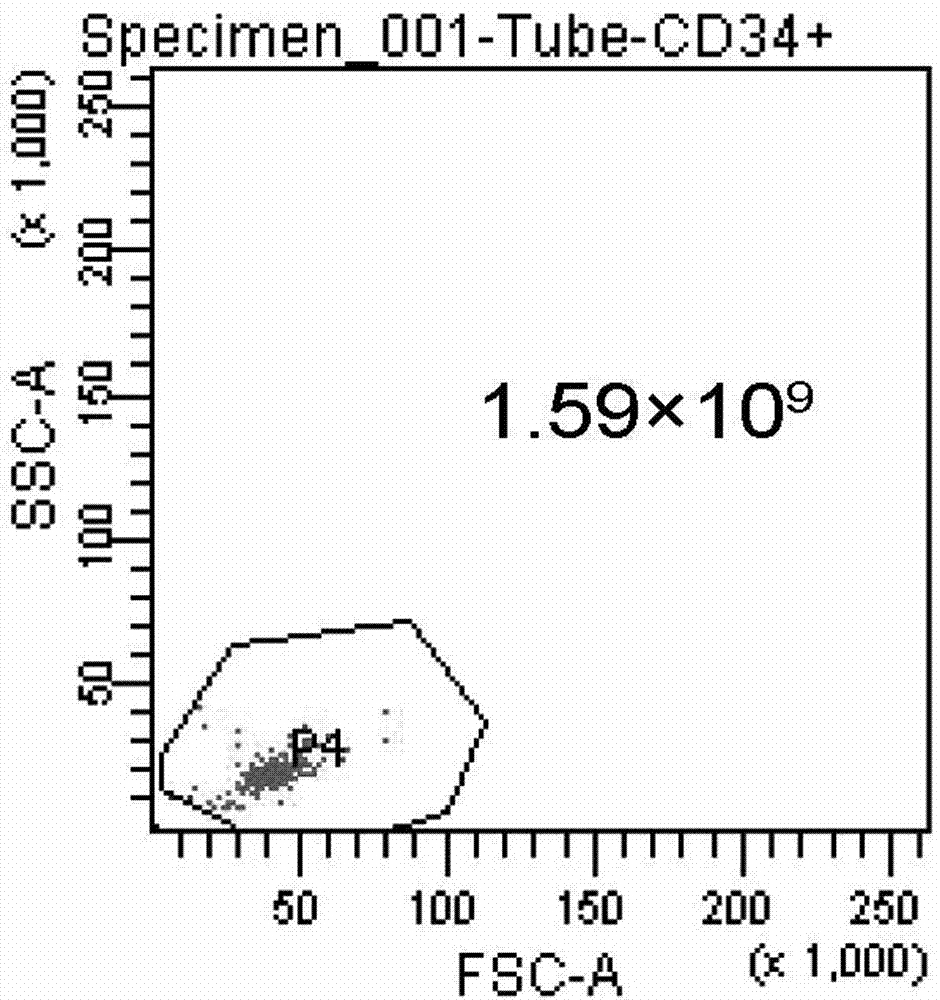
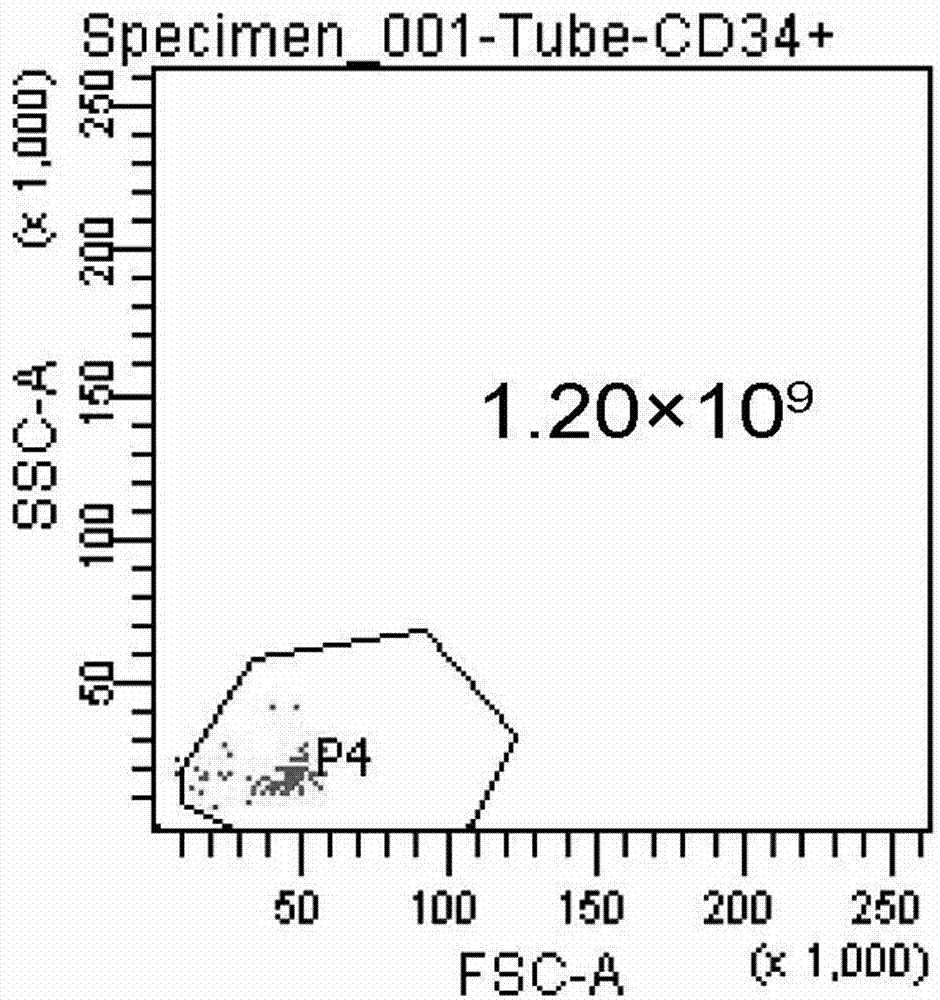
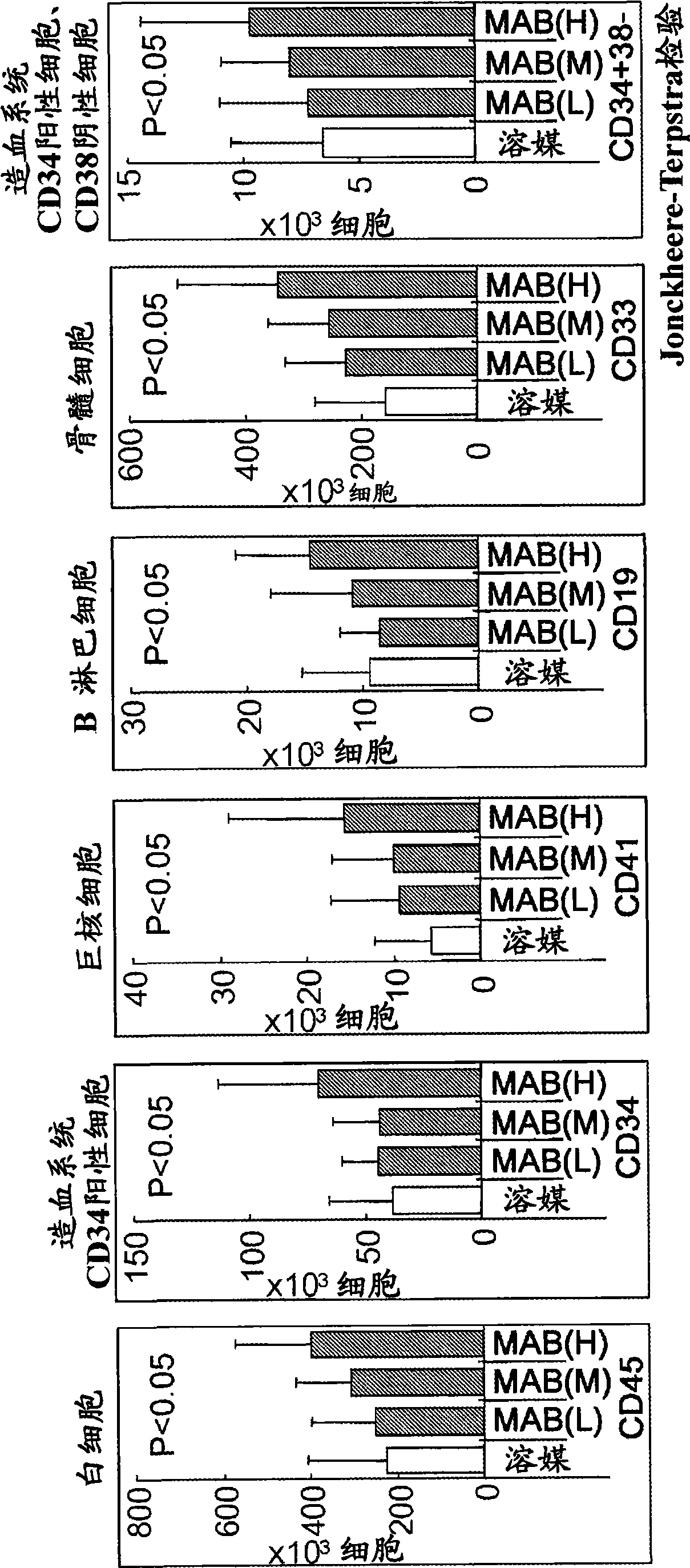
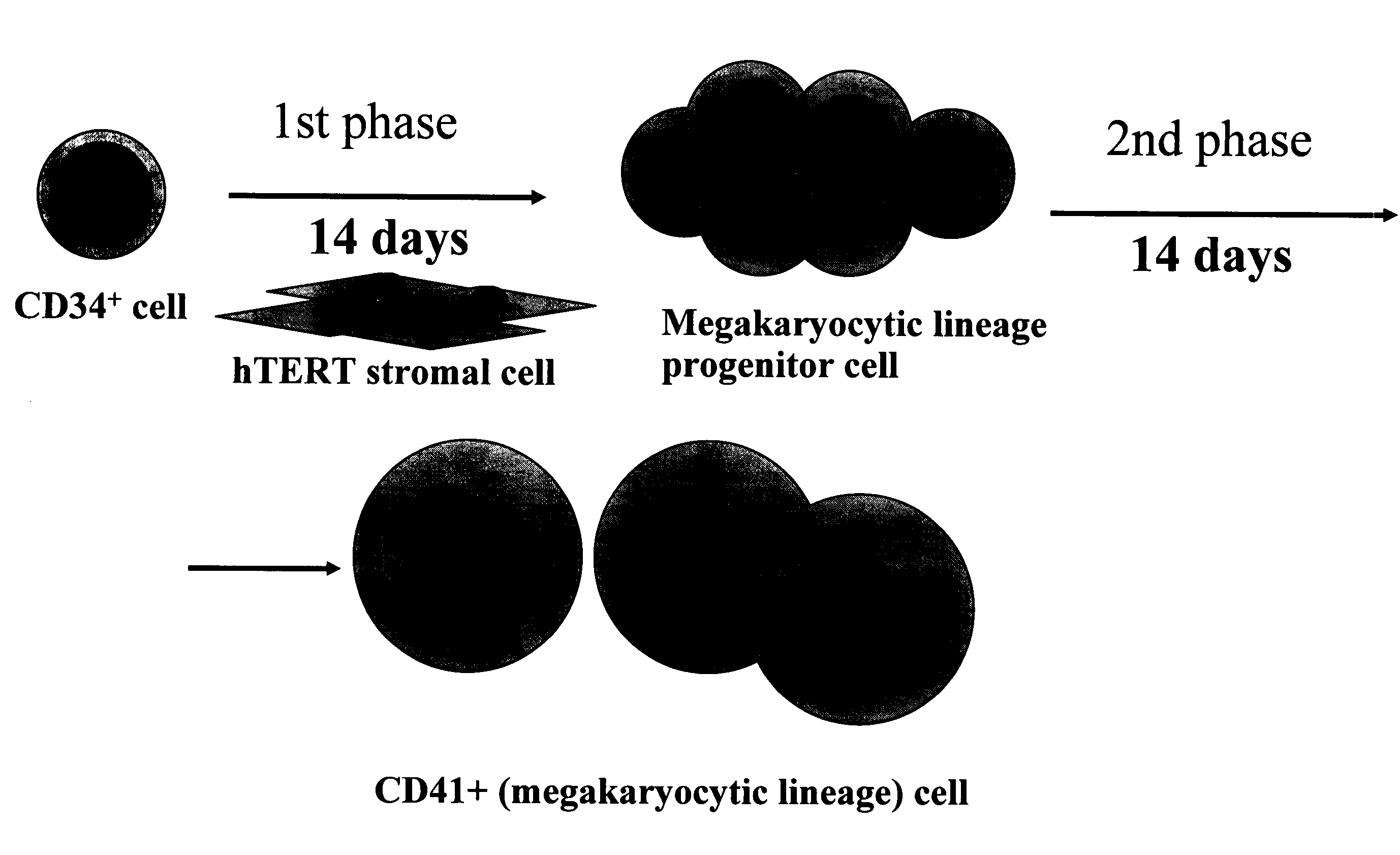
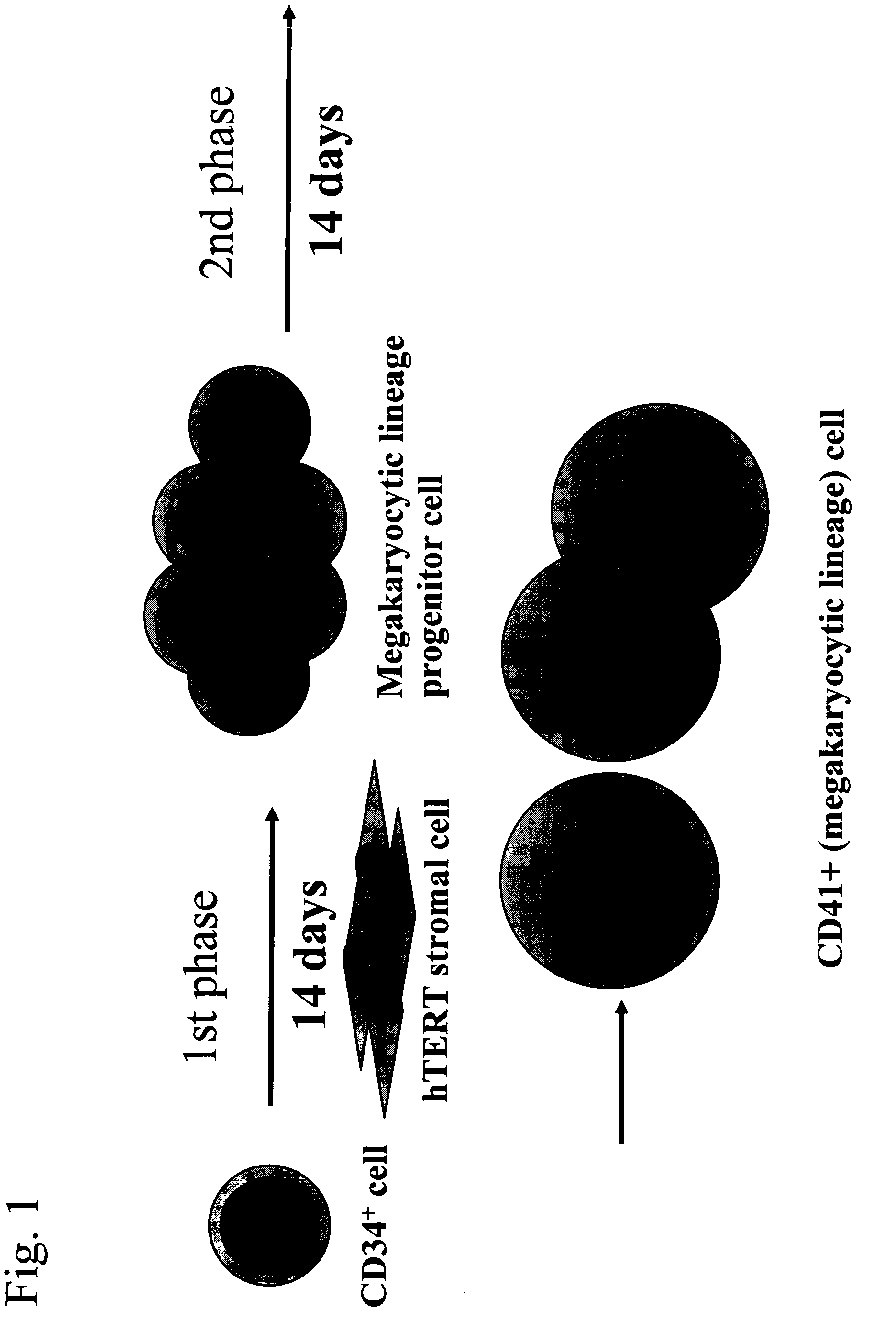
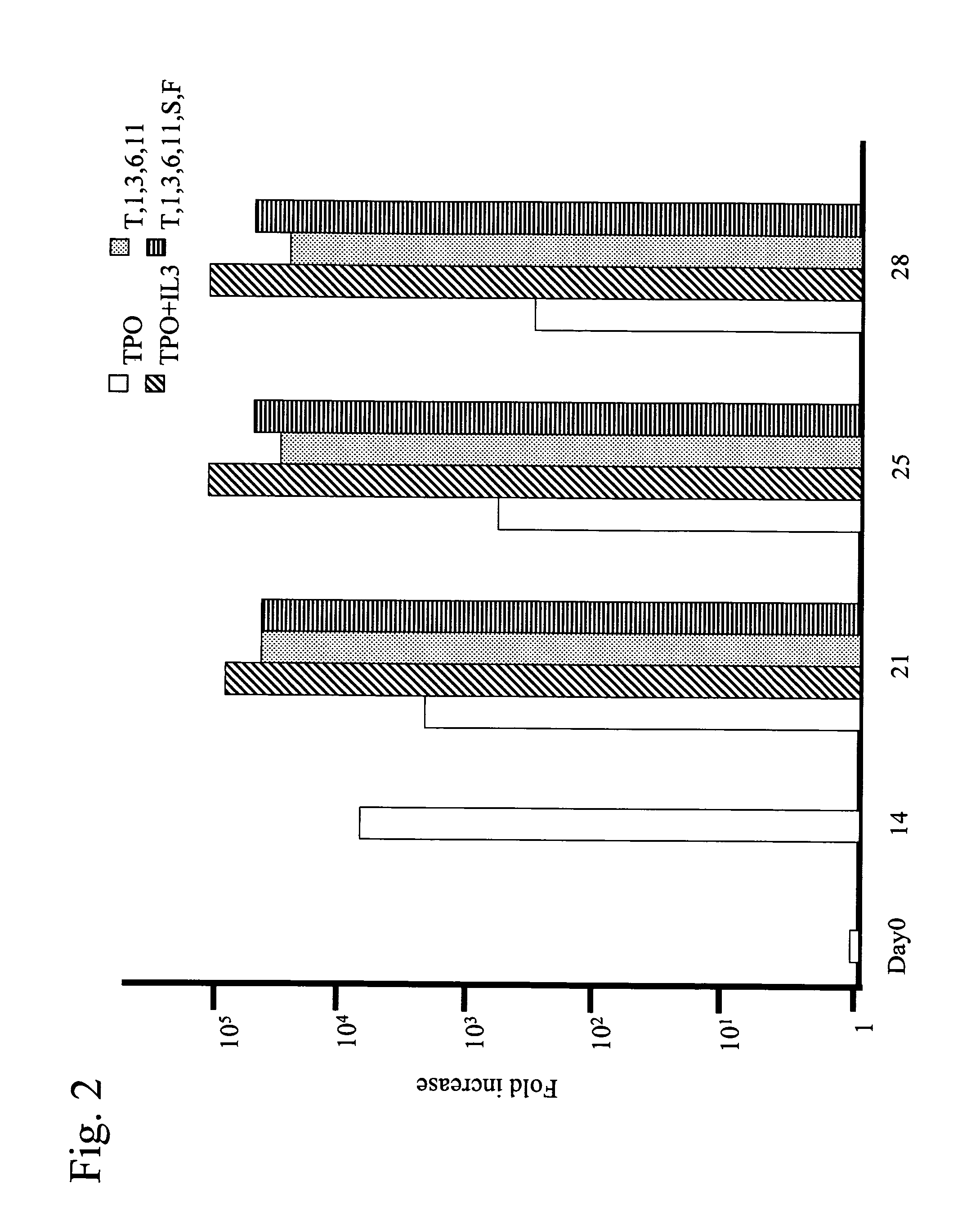
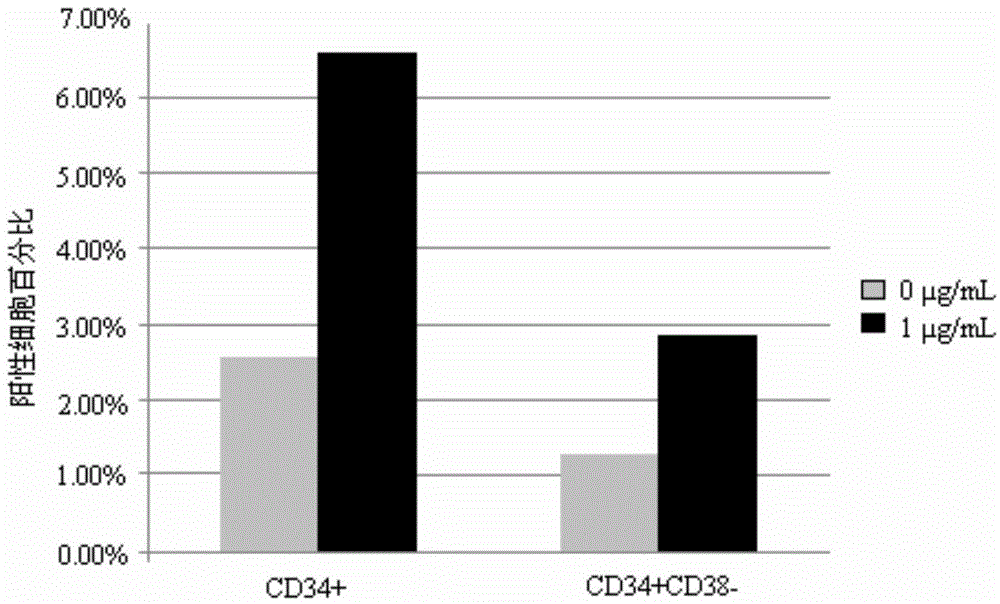
Differentiation of Cd34 Positive Cell to Megakaryocyte and Multiplication
InactiveUS20080233643A1Effectively lead to differentiationSkeletal/connective tissue cellsBlood/immune system cellsStromal cellCytokine
An object of the present invention is to provide a method for more efficiently differentiating CD34+ cells derived from umbilical cord blood into megakaryocytic lineage cells and for generating platelets. Coculture of CD34+ cells derived from umbilical cord blood with immortalized stromal cells in the presence of cytokines has enabled cell proliferation up to the 100,000-fold level, which has been impossible to achieve to date.
Owner:RENOMEDIX INST
Applications of CAPE (Caffeic Acid Phenylethyl Ester) in culturing hematopoietic stem/progenitor cells in vitro
The invention relates to applications of CAPE (Caffeic Acid Phenylethyl Ester) in culturing hematopoietic stem / progenitor cells in vitro. The CAPE with certain concentration is added in an in-vitro hematopoietic stem / progenitor cell expansion system, the ratio of the expanded human umbilical cord blood mononuclear cells or expanded hematopoietic stem / progenitor cells in CD34 positive cells is obviously increased, the total colony number is remarkably increased, so that the in-vitro expansion of the hematopoietic stem / progenitor cells can be effectively promoted, and the in-vitro expansion efficiency of the hematopoietic stem / progenitor cells can be enhanced.
Owner:FIELD OPERATION BLOOD TRANSFUSION INST OF PLA SCI ACAD OF MILITARY
A kind of preparation method of placental hematopoietic stem cell
ActiveCN109337871BNo damageHelp dissociationCell dissociation methodsDead animal preservationMedicinePlacenta
The invention belongs to the field of biotechnology, and specifically relates to a method for preparing placental hematopoietic stem cells, comprising the following steps: (1) placental pretreatment; (2) placenta perfusion once; (3) placenta perfusion twice; (4) placenta three times Perfusion; (5) mixed centrifugation; (6) preliminary purification of placenta; (7) cryopreservation of hematopoietic stem cells; this preparation method can increase the number of isolated CD34 positive cells and cell viability, and the reagents used have no potential risks. It can be applied clinically and reduces the cost of experiments.
Owner:山东康华生物医疗科技股份有限公司
Coated implant
InactiveUS8637062B2Reliable and controlled processSettlement of epithelial cells on the surface is promotedStentsSugar derivativesParylene coatingOligopeptide
Implant provided with a coating, with the implant being provided with an amino-functionalized parylene coating, an oligonucleotide and / or an oligopeptide having a specific bonding affinity with CD34-positive cells.
Owner:HAN BOCK SUN
Method for preparing CD34 positive cells from umbilical cord mesenchymal stem cells
ActiveCN106434557BSuccessful separationSuccessfully and efficiently separateMammal material medical ingredientsSkeletal/connective tissue cellsMesenchymal stem cellUmbilical cord
The invention relates to a method for preparing CD34 positive cells from umbilical cord mesenchymal stem cells. The method specifically comprises the following steps: (a) providing mesenchymal stem cells from an umbilical cord; (b) performing primary culture on the mesenchymal stem cells; (c) performing subculture on the mesenchymal stem cells; (d) in a subculture process, adding an inductive agent in a culture medium for inducing the mesenchymal stem cells; (e) obtaining the CD34 positive cells. The invention also relates to cell products of the CD34 positive cells, prepared through the method and application of the cell products. The method disclosed by the invention has excellent technical effects described in the description.
Owner:BOYALIFE
Technology for promoting direct trans-differentiation of umbilical cord blood CD34 positive cells into mesenchymal stem cells
InactiveCN107022522AEasy and safe conversionFar-reaching medical application valueSkeletal/connective tissue cellsBlood/immune system cellsSurface markerGenetic engineering
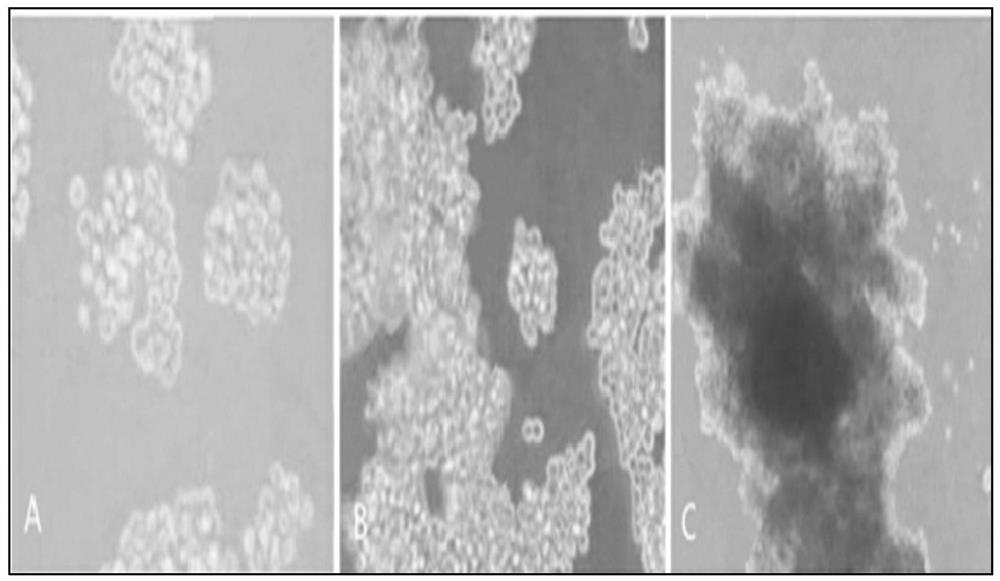
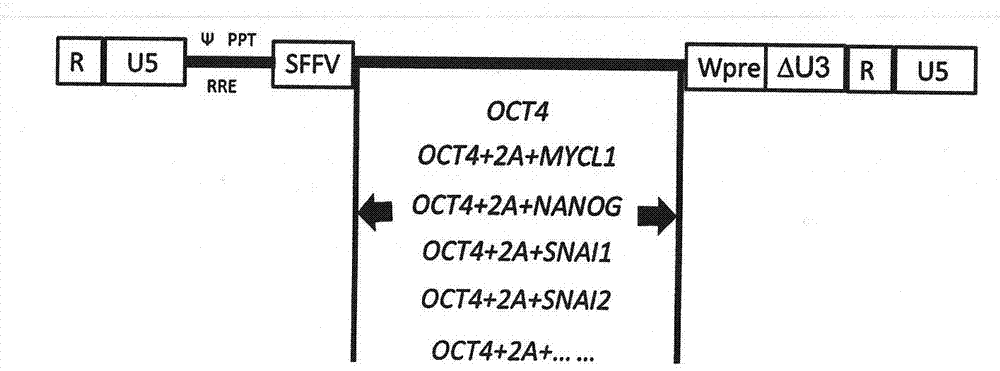
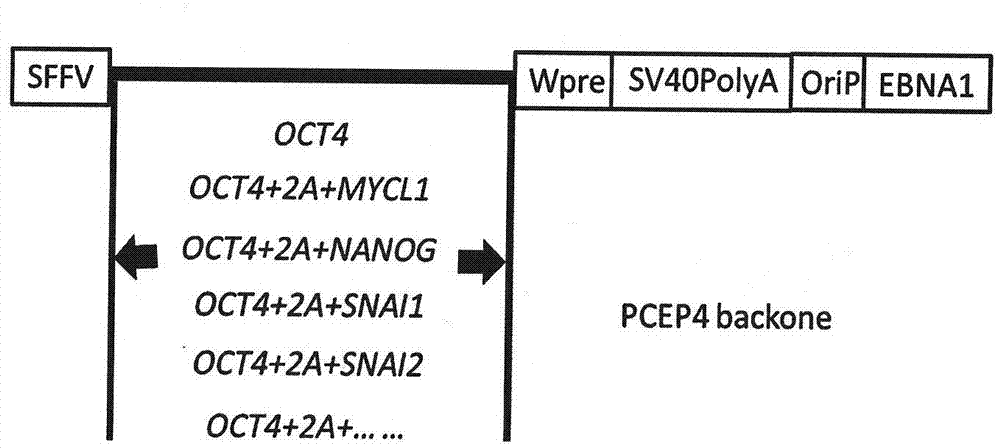
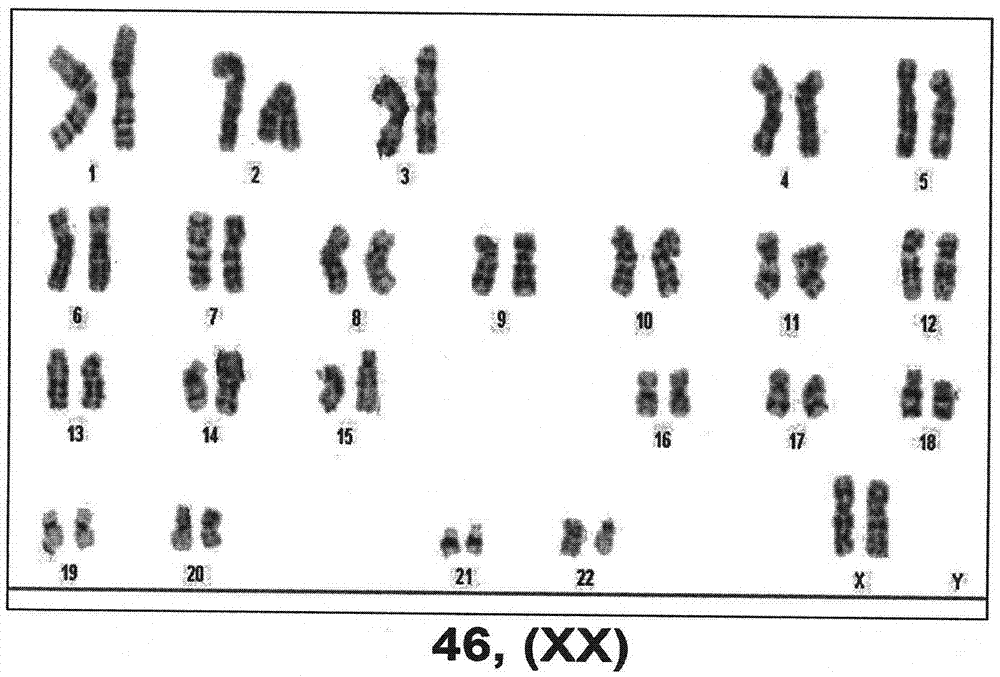
The invention relates to a technology for promoting direct trans-differentiation of umbilical cord blood CD34 positive cells into mesenchymal stem cells, and belongs to the technical field of bioengineering. With the application of the genetic engineering technology provided by the invention, efficient direct trans-differentiation of the umbilical cord blood CD34 positive cells from hematopoietic stem cells can be promoted, so that iMSCs can be obtained; the hematopoietic stem cells, which are co-cultured with a certain composition for enough time, can be transformed into MSCs through trans-differentiation; the composition includes at least one transcription factor selected from OCT4, NANOG, MYCL1, SNAI1, SNAI2 and TWIST1; and the composition can also include one or more cell factors disclosed by the invention and other compounds. With the application of the technical method provided by the invention, transient expression of the related transcription factors in the umbilical cord blood CD34 positive cells can be achieved by virtue of a virus infection method, and in the combination with an MSCs inducing medium, 1*10<9> MSCs can be obtained from 1*10<6> umbilical cord blood CD34 positive cells through the trans-differentiation, and the iMSCs cells, obtained from the trans-differentiation, are represented in the form of the MSCs and can express surface markers of the MSCs cells, such as CD29, CD44, CD73 and CD90.
Owner:溯源生命科技股份有限公司
Method of detecting leukemic cell
InactiveUS8617804B2Conveniently obtainMicrobiological testing/measurementBiological material analysisBlood specimenSeverity/Intensity
[Problems] To provide a marker which is useful in diagnosing leukemia and a method of using the same.[Means for Solving Problems] A method of detecting a leukemia cell which comprises detecting a CD34-positive cell being negative in DOCK180 expression from a blood specimen. According to this method, a DOCK180 expression pattern specific to a tumor cell in leukemia, in particular, acute leukemia is confirmed so that information which is useful in diagnosing leukemia and monitoring and determining the severity and degree of recovery of the same by a doctor can be obtained by a convenient procedure using blood specimens and thus provided.
Owner:HOKKAIDO UNIVERSITY
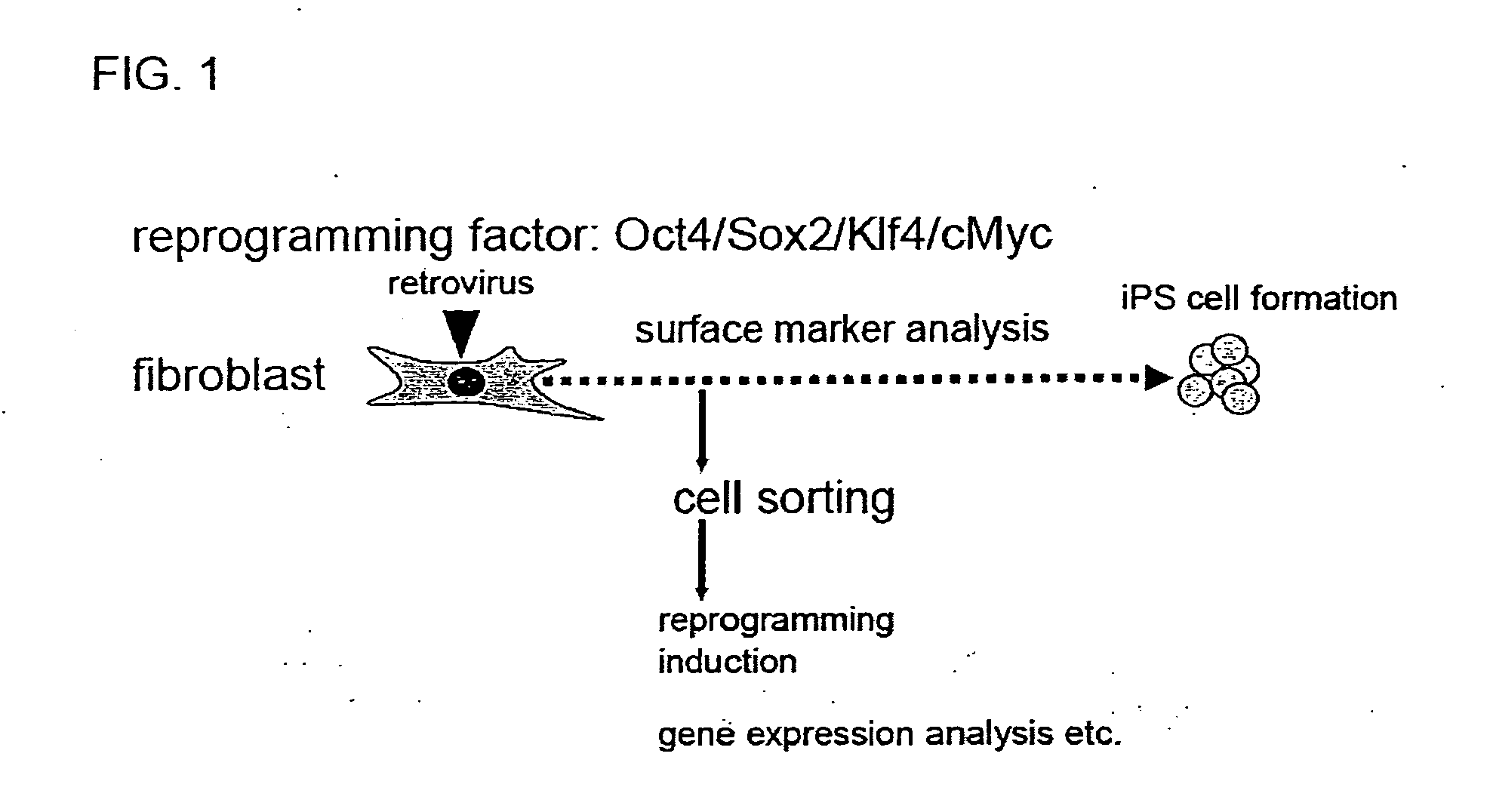
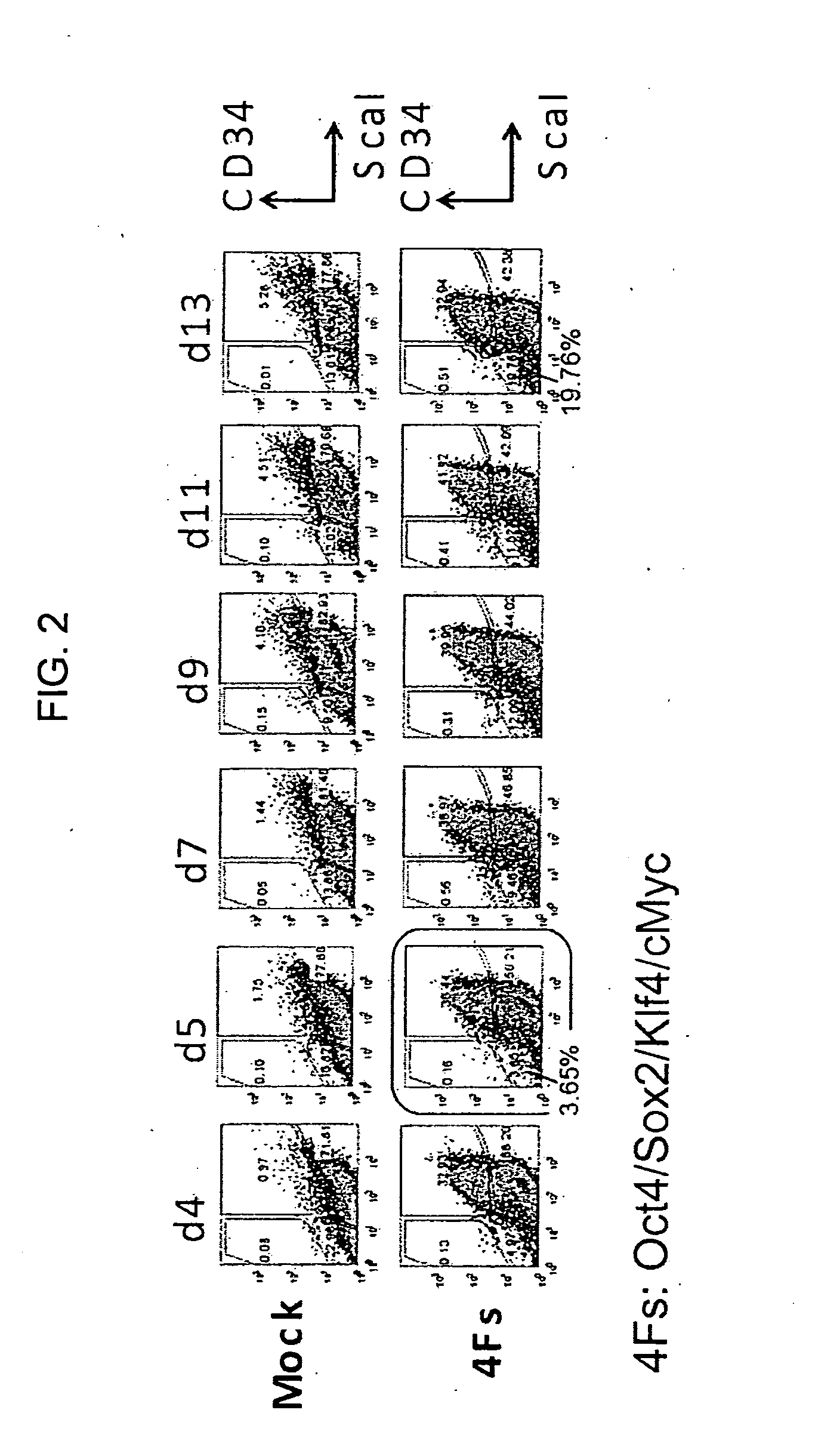
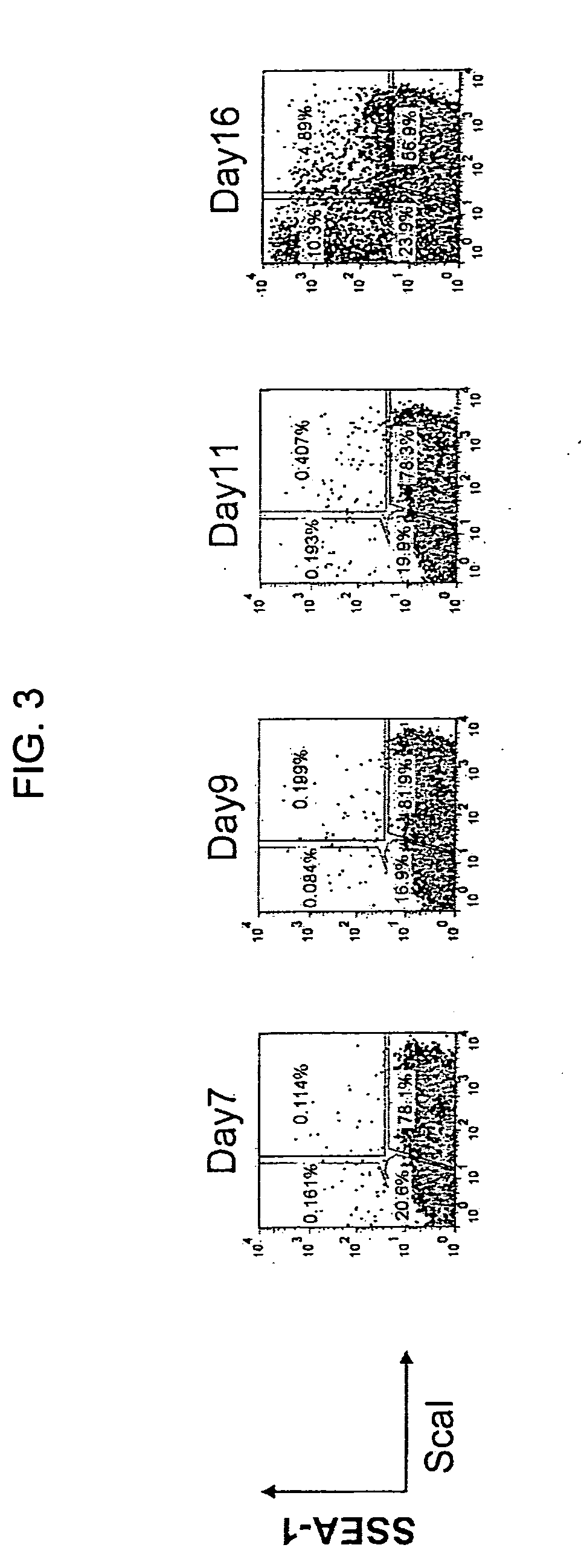
Method for producing induced pluripotent stem cells, cardiomyocytes or precursor cells thereof
InactiveUS20150337266A1Easily reprogrammedReduce riskGenetically modified cellsArtificial cell constructsCardiac muscleNeoplasm
Nuclear reprogramming substances are contacted with a somatic cell and, after culture, cell population is fractionated based on the expression of Sca-1 or EpCam and CD34. By sorting Sca-1-negative CD34-negative cells or EpCam-positive CD34-negative cells, a cell population having high possibility of iPS cell formation can be selected. On the other hand, Sca-1-positive CD34-positive cells or EpCam-negative CD34-positive cells are useful as a source of myocardial cell or a progenitor cell thereof having a low risk of tumor formation.
Owner:KYOTO UNIV
Cell therapy agents are used to alleviate or improve the method of vascular lesions
ActiveCN110840914BSuccessful separationSuccessfully and efficiently separateUnknown materialsBlood/immune system cellsArterial stenosisCell therapy
The invention relates to a method for alleviating or improving vascular lesions by a cell therapy agent of CD34 positive cells. In one aspect, the cell therapy compositions involved include CD34 + Cells and pharmaceutically acceptable carriers, for example, containing: CD34 + cells, sodium chloride, water for injection, CD34 + The density of cells is 1×10 6 A / mL~5×10 6 Individual / mL, the mass volume percentage of sodium chloride is 0.8% to 1.0% or 0.9%. It also relates to the preparation method of the cell therapy agent comprising CD34 positive cells, and the use of the cell therapy composition in the preparation of medicines for relieving or improving vascular disease, wherein the vascular disease is caused by stenosis and occlusion of limb arteries or diabetes Peripheral arterial disease; preferred limb arterial stenosis and occlusion or lower extremity arterial disease caused by diabetes. The method of the present invention exhibits excellent technical effects as described in the present invention.
Owner:BOYALIFE
Method for inducing and accelerating cells
Owner:VER VU WINDESHEIM +1
Method of amplifying hematopoietic stem cells of umbilical cord blood in vitro
InactiveCN107446890ASkeletal/connective tissue cellsBlood/immune system cellsMesenchymal stem cellCell state
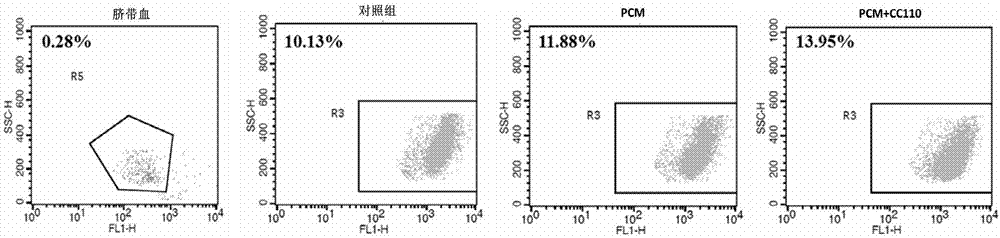
The invention relates to a method of amplifying hematopoietic stem cells of umbilical cord blood in vitro and belongs to the technical field of biomedicines. The method amplifies the hematopoietic stem cells of umbilical cord blood in vitro by means of CBMC separation of a mononuclear cell of umbilical cord blood, CD34 positive cell enrichment and CD34 positive cell expansion, and the amplified hematopoietic stem cells of umbilical cord blood are detected relatively. According to the method provided by the invention, the hematopoietic stem cells of umbilical cord blood can be amplified in a multiple fold manner, and the cells meet the clinical application standard. By expansion in vitro, the quality of implanting the hematopoietic stem cells of umbilical cord blood for clinical use of an adult is met. No treatment methods affecting the cell state are employed, and a condition culture medium of mesenchymal stem cells is used for amplifying the hematopoietic stem cells. Compared with a control group, the method can raise the proportion of the D34 positive hematopoietic stem cells from 0.28% to 13.95%, and has a good application prospect.
Owner:YUNNAN SHUNXI REGENERATION MEDICAL ENG CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com