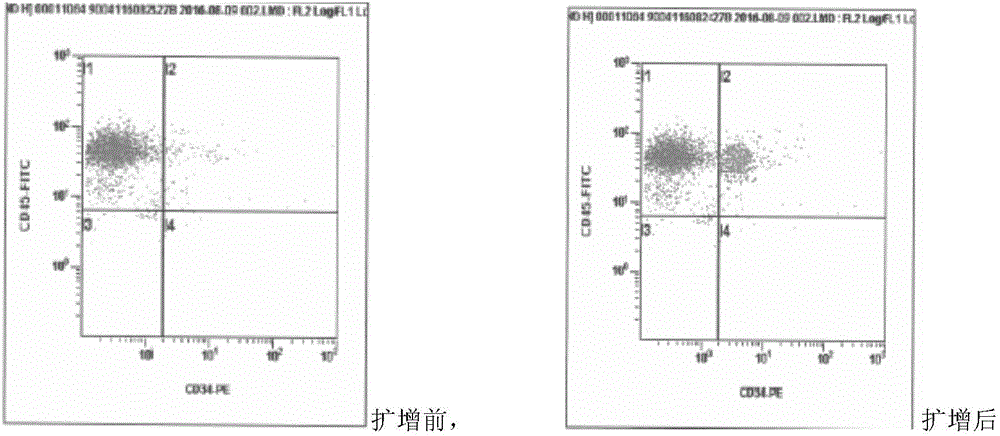
Method for separating hematopoietic stem cells from umbilical cord blood and amplifying CD34 positive cells
A technology of hematopoietic stem cells and positive cells, applied in the field of CD34+ cell preparation, can solve the problems of limited supply of human umbilical cord blood, limited number of CD34+ cells, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0120] Example 1. Preparation of CD34 positive cells from umbilical cord blood hematopoietic stem cells
[0121] The formula of the hematopoietic stem cell culture system used in this example is: human low-density lipoprotein (Human LDL) 75 μg / ml, erythropoietin (EPO) 5ng / ml, interleukin 3 (IL-3) 1500IU / ml, stem cell factor (SCF) 25ng / ml, an appropriate amount of animal component-free medium (StemSpan-ACF) was added to the full volume of the dosing solution.
[0122] In this example, after the hematopoietic stem cells were isolated from the collected umbilical cord blood, they were first cryopreserved and resuscitated, and then CD34 + Expansion of cells so that cryopreservation-resuscitation processing does not lose / damage cord blood hematopoietic stem cells and in some cases large cord blood samples can be collected for CD34 as needed + Expansion of cells.
[0123] 1. Cryopreservation of umbilical cord blood hematopoietic stem cells
[0124] After the fresh umbilical cor...
Embodiment 2
[0142] Example 2. Preparation of CD34 positive cells from umbilical cord blood hematopoietic stem cells
[0143] The formula of the hematopoietic stem cell culture system used in this example is: human low-density lipoprotein (Human LDL) 25 μg / ml, erythropoietin (EPO) 15ng / ml, interleukin 3 (IL-3) 500IU / ml, stem cell factor (SCF) 75ng / ml, and an appropriate amount of animal component-free medium (StemSpan-ACF) was added to the full volume of the dosing solution.
[0144] In this example, cryopreservation and resuscitation operations are not performed, but the collected cord blood is directly subjected to CD34 + Expansion of cells.
[0145] (1). Separation of umbilical cord blood hematopoietic stem cells: after the fresh umbilical cord blood is collected, separate umbilical cord blood hematopoietic stem cells to obtain nucleated cells;
[0146] (2). Use a disposable sterile syringe to transfer 5ml of cord blood hematopoietic stem cells obtained in step (1) to a 50ml sterile...
Embodiment 3
[0158] Example 3, Preparation of CD34 positive cells from umbilical cord blood hematopoietic stem cells
[0159] The formula of the hematopoietic stem cell culture system used in this example is: human low-density lipoprotein (Human LDL) 50 μg / ml, erythropoietin (EPO) 10 ng / ml, interleukin 3 (IL-3) 1000IU / ml, stem cell factor (SCF) 50ng / ml, and an appropriate amount of animal component-free medium (StemSpan-ACF) was added to the full volume of the dosing solution.
[0160] In this example, after the hematopoietic stem cells were isolated from the collected umbilical cord blood, they were first cryopreserved and resuscitated, and then CD34 + Expansion of cells.
[0161] 1. Cryopreservation of umbilical cord blood hematopoietic stem cells
[0162] After the fresh umbilical cord blood is collected, it passes through the AXP Automatic Separation System (AXP Auto Xpress TM Platform) to obtain nucleated cells, and then add cell freezing solution (65 parts of DMEM-F12, 10 parts ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com