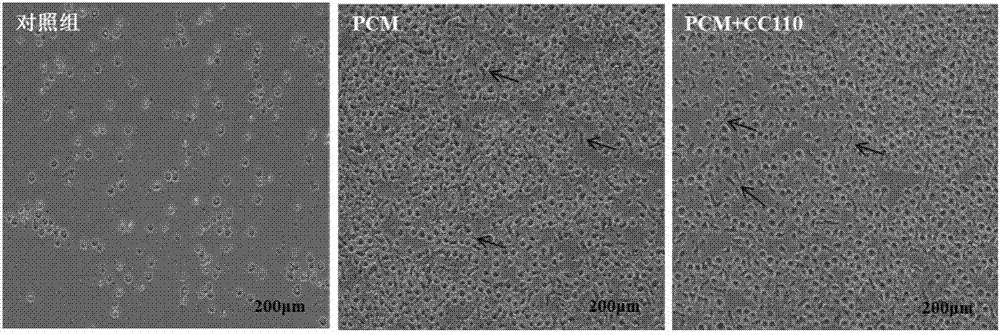
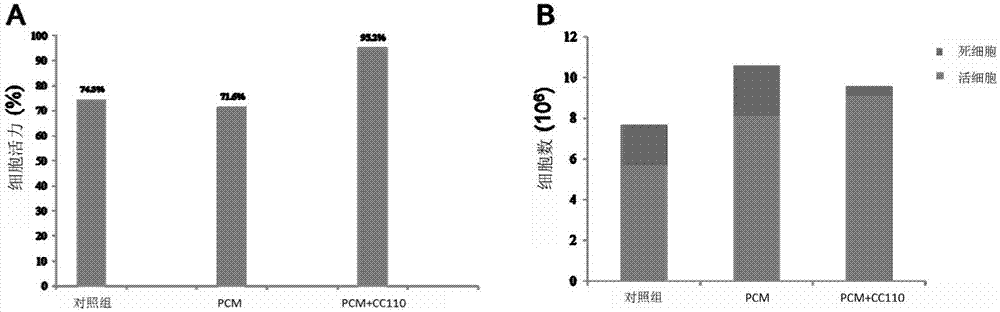
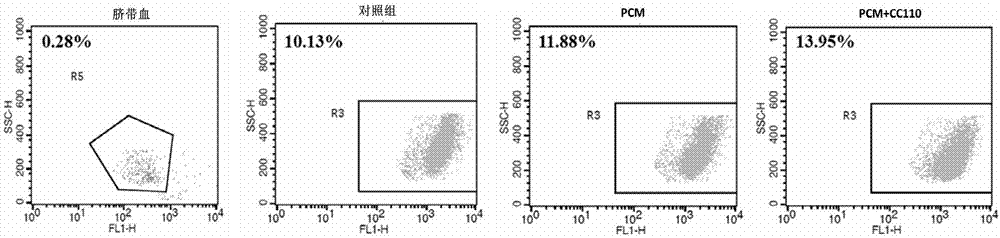
Method of amplifying hematopoietic stem cells of umbilical cord blood in vitro
A technology for in vitro expansion of hematopoietic stem cells, applied in the field of in vitro expansion of umbilical cord blood hematopoietic stem cells, can solve problems such as low expansion efficiency that cannot meet clinical needs, insufficient number of umbilical cord blood hematopoietic stem cell transplants, and inability to expand umbilical cord blood hematopoietic stem cells , to achieve the effect of good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] A method for expanding umbilical cord blood hematopoietic stem cells in vitro, comprising the steps of:
[0044] Step (1), centrifuge cord blood at 650g for 15 minutes to remove plasma, then add an equal volume of normal saline to the remaining blood cells to dilute the blood cells;
[0045] Step (2), take the centrifuge tube added with Ficoll-diatrizoate solution, and then add the blood cells diluted in step (1) along the wall of the centrifuge tube to make the boundary clear. The volume ratio of sucrose-diatrizoate meglumine solution is 4:3. Centrifuge at 1800 rpm for 30 minutes at 18°C, then take the buffy coat layer and wash it twice with normal saline. The volume of normal saline used each time is white Three times the volume of the membrane layer, cleaned by centrifugation, the centrifugation speed is 1200 rpm, and the centrifugation time is 5 minutes each time to obtain CBMC (cord blood mononuclear cells);
[0046] Wherein, every 100mL polysucrose-diatrizoate so...
Embodiment 2
[0055] A method for expanding umbilical cord blood hematopoietic stem cells in vitro, comprising the steps of:
[0056] Step (1), centrifuge cord blood at 700g for 20 minutes to remove plasma, then add an equal volume of normal saline to the remaining blood cells to dilute the blood cells;
[0057] Step (2), take the centrifuge tube added with Ficoll-diatrizoate solution, and then add the blood cells diluted in step (1) along the wall of the centrifuge tube to make the boundary clear. The volume ratio of sucrose-diatrizoate meglumine solution is 4:3, centrifuge at 2000 rpm for 45 minutes at 20°C, then take the buffy coat layer and wash it with normal saline 2~3 times, the volume of normal saline used each time 4 times the volume of the buffy coat, cleaned by centrifugation, the centrifugation speed is 1500 rpm, each centrifugation time is 8 minutes, and CBMC (cord blood mononuclear cells) are obtained;
[0058] Wherein, every 100mL polysucrose-diatrizoate solution contains po...
Embodiment 3
[0067] A method for expanding umbilical cord blood hematopoietic stem cells in vitro, comprising the steps of:
[0068] Step (1), centrifuge cord blood at 660g for 18 minutes to remove plasma, then add an equal volume of normal saline to the remaining blood cells to dilute the blood cells;
[0069] Step (2), take the centrifuge tube added with Ficoll-diatrizoate solution, and then add the blood cells diluted in step (1) along the wall of the centrifuge tube to make the boundary clear. The volume ratio of sucrose-diatrizoate meglumine solution is 4:3, centrifuge at 1900 rpm for 39 minutes at 19°C, then take the buffy coat layer and wash it twice with normal saline, the volume of normal saline used each time is white 3.5 times the volume of the film layer, cleaned by centrifugation, the centrifugation speed is 1300 rpm, and the centrifugation time is 7 minutes each time to obtain CBMC (cord blood mononuclear cells);
[0070] Wherein, every 100mL polysucrose-diatrizoate solution...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com