Preparation method and preservation method of clinical application-level placental hematopoietic stem cells
A technology of hematopoietic stem cells and clinical application, which is applied in the field of preparation of clinical application-grade placental hematopoietic stem cells and preservation of placental hematopoietic stem cells. It can solve the problems of large processing capacity, patient infection, and maintenance, and achieve reduced digestive enzyme consumption and high cell activity. , handle large volume effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1: Acquisition of placental hematopoietic stem cells
[0038] 1. Collect the placenta of normal vaginal delivery or caesarean section under aseptic conditions, pre-treat immediately, and then separate two umbilical arteries and one umbilical vein, and insert a puncture needle connected to a syringe or a constant-flow pump or a pediatric scalp needle into the umbilical cord vein. Use 300ml of DEME culture solution containing penicillin and streptomycin (the concentration of antibiotics is 200mg / L, and the ratio of penicillin and streptomycin added is 1:1) as the cleaning solution to perfuse the placental villi vessels, and collect the cleaning fluid after perfusion. liquid;
[0039]2. Enzyme digestion. In order to better illustrate the practicability of the present invention, three different digestion methods are listed here for specific description, but the enzyme digestion method of the present invention is not limited thereto:
[0040] Enzyme digestion method...
Embodiment 2
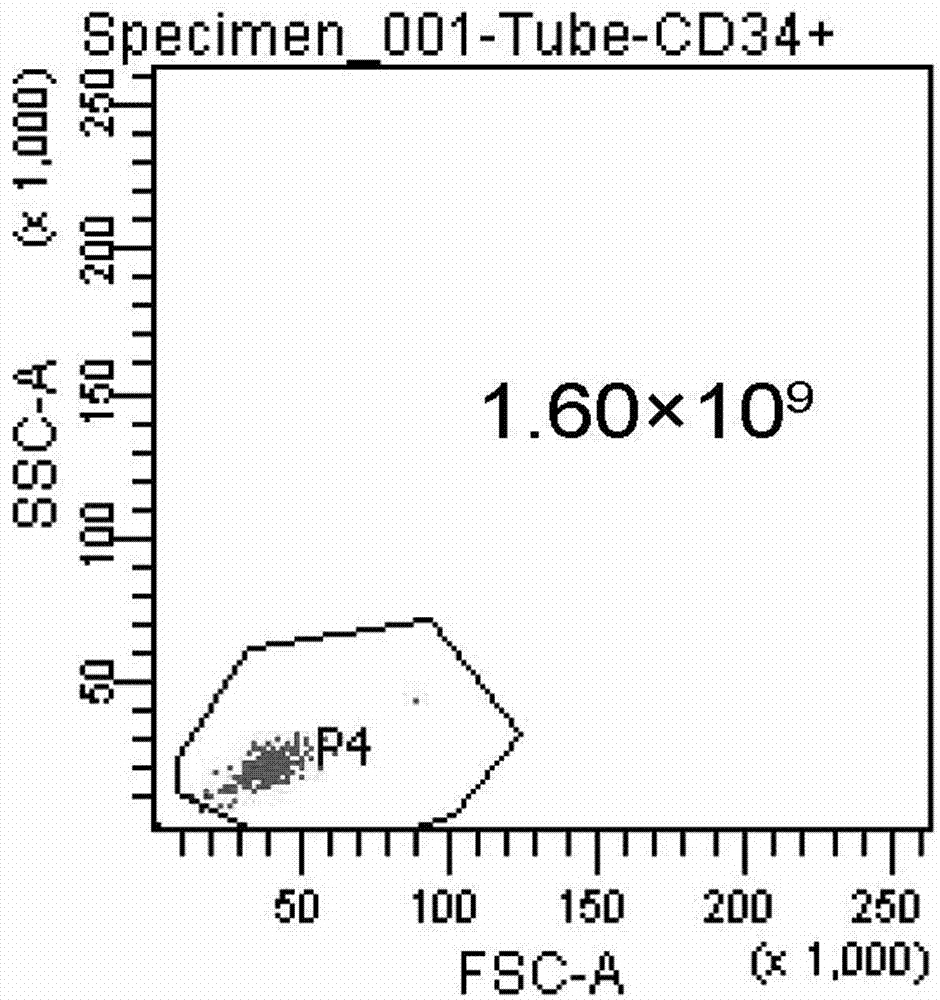
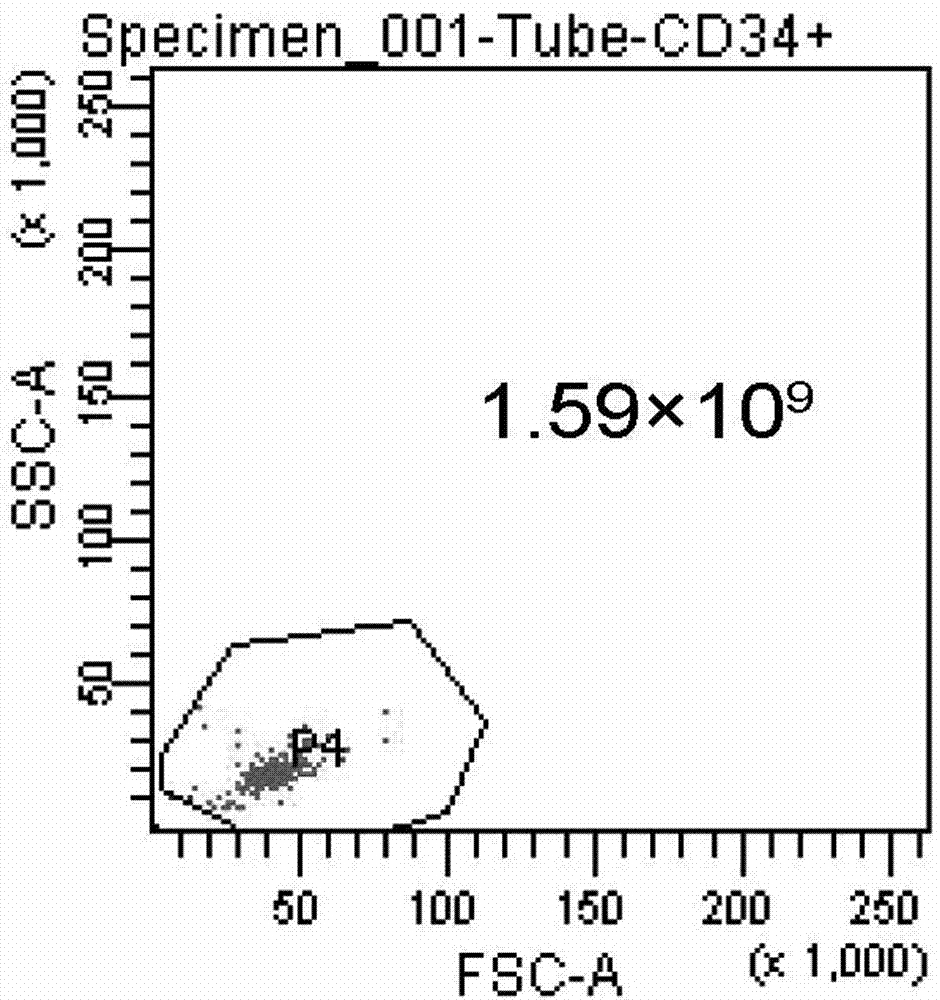
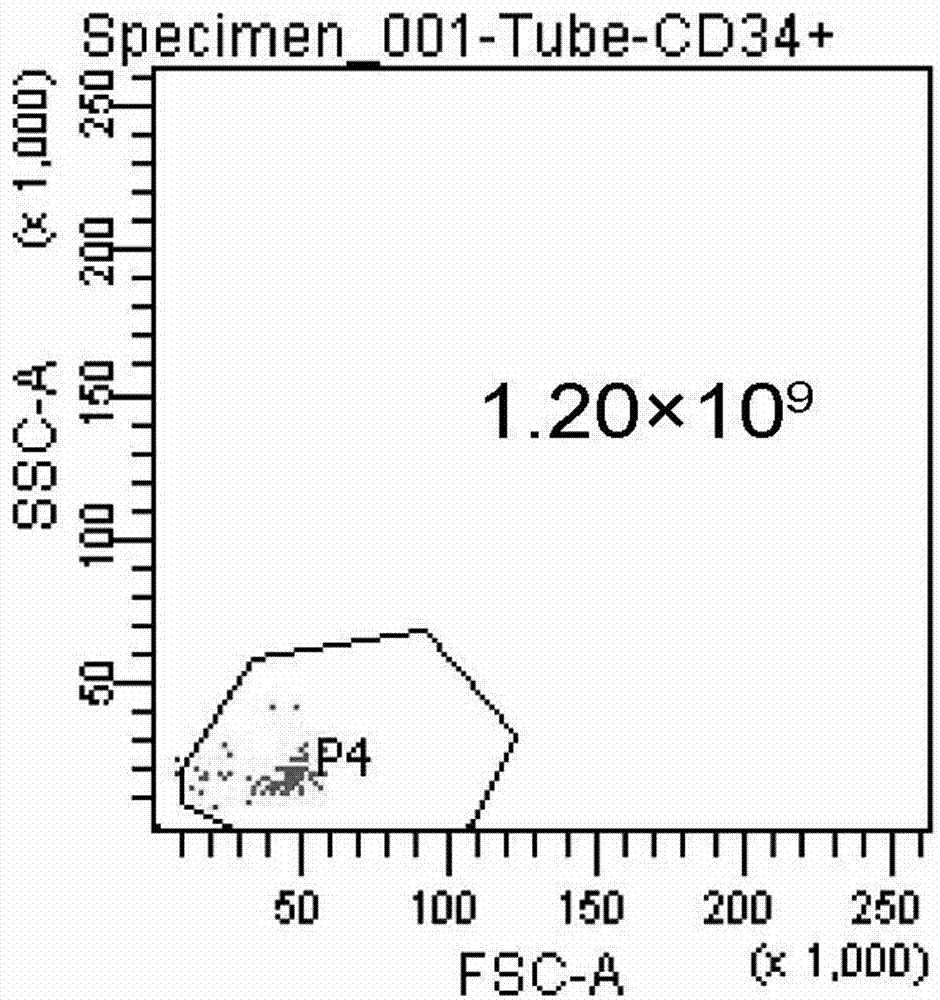
[0049] Example 2: Detection of placental hematopoietic stem cells
[0050] 1. After the pretreatment of the freshly delivered placenta and before the preparation of placental hematopoietic stem cells: Hepatitis virus and corresponding antibody detection, AIDS antibody detection, syphilis antibody detection;
[0051] 2. At the same time as placental hematopoietic stem cells are prepared: bacteria and fungi are tested;
[0052] 3. After the placental hematopoietic stem cells are prepared: Quantitative detection of hematopoietic stem cells (CD34) and activity of hematopoietic stem cells (trypan blue staining).
Embodiment 3
[0053] Example 3: Cryopreservation of placental hematopoietic stem cells
[0054] 1. Prepare cryopreservation solution (10% DMSO + 5% human recombinant albumin + DEME culture solution): take a centrifuge tube, add 9ml DEME culture solution, then slowly add 6.75ml DMSO dropwise to the centrifuge tube, while adding After oscillating and shaking well, cover the lid, tighten it, and place it in a refrigerator at 4°C for pre-cooling. After the pre-cooling is completed, slowly add 6.75ml human recombinant albumin (purchased from Mitsubishi Pharmaceutical Group Co., Ltd. of Japan) dropwise, and shake while adding Mix well to avoid precipitation, cover the lid, tighten it, and pre-cool in a 4°C refrigerator for later use;
[0055] 2. Take out the pre-prepared cryopreservation solution, slowly drop it into the cell suspension, add 10ml to each tube, shake while dropping, mix well quickly, cover the lid, and pre-cool in a 4°C refrigerator;
[0056] 3. Take out the pre-cooled cell suspe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com