Application of allyl-containing monocarbonyl curcumin analogs in preparing antiinflammatory drugs
A technology of allyl phenyl and medicinal salts, applied in the field of medicinal chemistry, can solve the problems of excessive metabolism, low bioavailability, low activity of curcumin, and limited application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] The synthesis of embodiment 1 compound
[0047] Preparation of intermediate 4-allyloxybenzaldehyde or 4'-(3-methyl-2-butenyloxy)benzaldehyde (7): Weigh 5 g (40.9 mmol) of p-hydroxybenzaldehyde and potassium carbonate 11.3 g (81.8mmol), it was dissolved in 20ml of acetone, stirred until dissolved, then added allyl bromide 9.9g (81.8mmol) or isopentenyl benzaldehyde 15.56g (81.8mmol), stirred at room temperature, TLC The reaction process was monitored, and the reaction was completed after about 5 hours. Spin the solvent with a vacuum pump, then extract with ethyl acetate, wash with saturated NaCl for 3 times, and wash the ethyl acetate layer with anhydrous NaCl 2 SO 4 After drying, the ethyl acetate layer was removed by rotary evaporation and concentrated to obtain a crude product, which was purified by silica gel column chromatography to obtain a yellow oily liquid 7.
[0048] Preparation of intermediate α, β-unsaturated ketone analog (9): Weigh 2 g (11.3 mmol) of com...
Embodiment 2
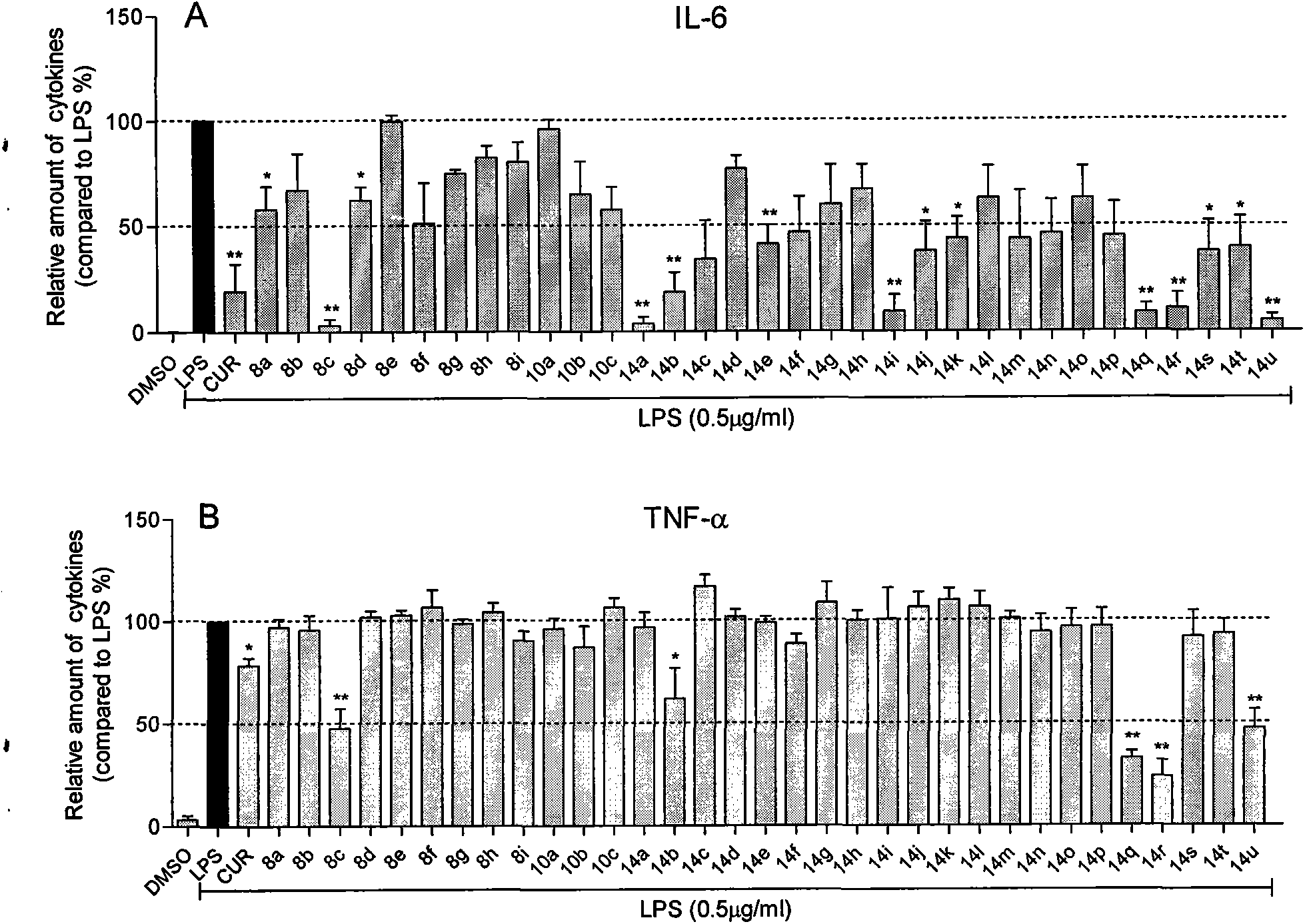
[0071] Inhibition of the compound of Example 2 on the release of inflammatory factors from macrophages stimulated by LPS
[0072] The preliminary anti-inflammatory activity of the compound in vitro was tested by using the compound to inhibit the release of inflammatory factors (TNF-α and IL-6) from RAW264.7 macrophages stimulated by LPS. The specific method is as follows: 1.2×10 6 RAW264.7 macrophages were cultured with DMEM medium at 37°C. After 24 hours, the medium was renewed, and the test compound (final concentration: 10 μM) was added for pretreatment for 2 hours, and then treated with 0.5 μg / mL LPS for 22 hours. hours, collect the culture fluid and use ELISA method to detect the content of TNF-α and IL-6; collect the cells to detect the total protein concentration, the ELISA results are divided by the corresponding total protein concentration, and the TNF-α and IL-6 in the LPS control group The content was calibrated to 100; each compound was tested three times, and the ...
Embodiment 3
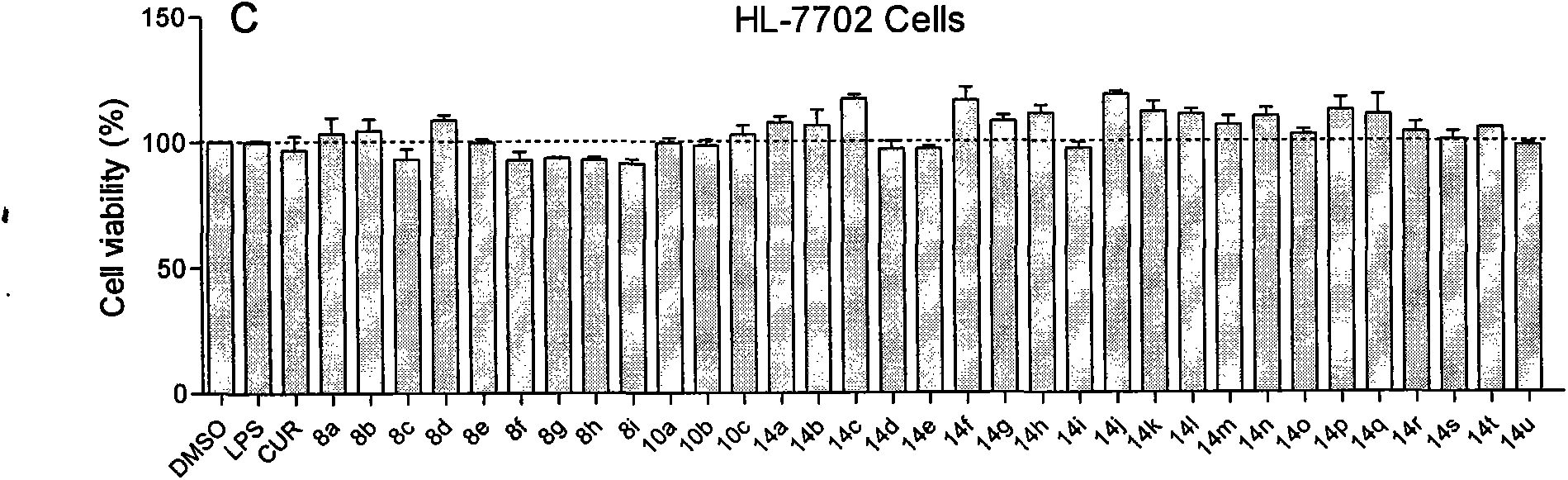
[0073] Example 3 Toxicity of Active Compounds to Human Normal Hepatocytes HL-7702
[0074] Spread HL7702 cells into 96-well plates at a density of 5000 cells per well, and use 1640 medium containing 5% heat-inactivated serum, 100 U / ml penicillin, and 100 μg / ml streptomycin at 37 °C containing 5%CO 2cultured in an incubator for 24 h. The test compound dissolved in DMSO was added to the culture medium with a final concentration of 20 μM, and the MTT assay was carried out after 72 hours of action. Add 25 μL of MTT (5 mg / ml) dissolved in physiological saline to each well and incubate for 3 h. Then the cells were lysed with 100 μL DMSO, and the OD value was detected with a microplate reader at a wavelength of 570 nm. For experimental data, see image 3 . All tested active compounds showed no toxicity to HL7702 cells.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap