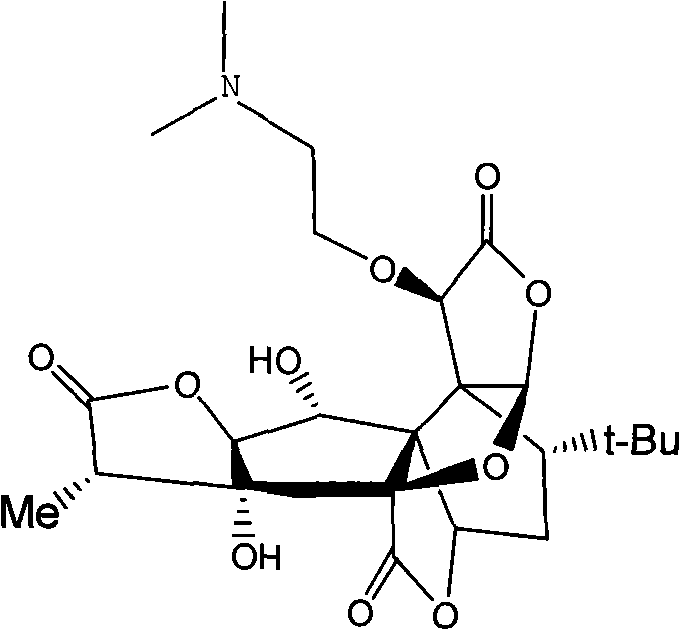
New application of Ginkgolide B derivative in medicament preparation
A technology of ginkgolide and its effect is applied in the new application field of 10-O-ginkgolide B, and can solve the problems such as the application of 10-O-(dimethylaminoethyl) ginkgolide B that has not been seen yet.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 110
[0015] The analgesic effect of embodiment 110-O-(dimethylaminoethyl) ginkgolide B
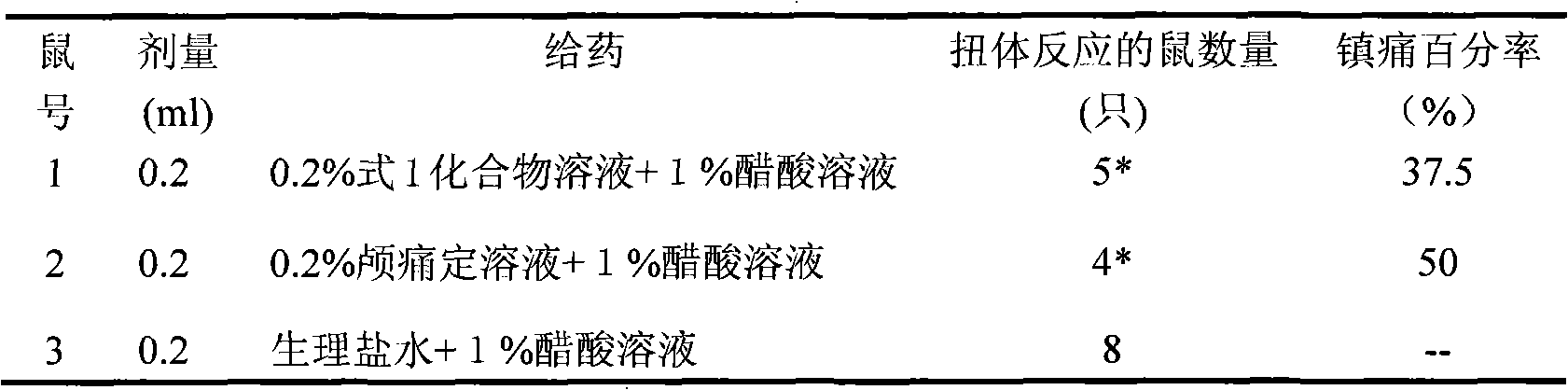
[0016] Take 30 mice weighing 18-22 g, half male and half male. Randomly divided into 3 groups, 10 in each group, numbered, and placed in a glass bell jar to observe the normal activities of the mice. The mice were injected intraperitoneally with 0.2% compound of formula 1 (0.2ml / 10g), 0.2% cranidinidine solution (0.2ml / 10g) and equal volume of normal saline respectively. After 30 minutes, each mouse was injected with 1% acetic acid solution 2ml / 10g intraperitoneally, and the number of mice with writhing reaction in each group was observed. Record the results in the table below, and calculate the analgesic percentage of analgesics by integrating all laboratory data.
[0017]
[0018] Table 1 formula 1 compound writhing reaction experiment result
[0019]
[0020] *p<0.01, compared with model group
[0021] It can be seen from Table 1 that the analgesic percentage of the compound of for...
Embodiment 210
[0021] It can be seen from Table 1 that the analgesic percentage of the compound of formula 1 is 37.5%, which has obvious analgesic effect. Anti-inflammatory test of fat-induced rat granuloma of embodiment 210-O-(dimethylaminoethyl) ginkgolide B
[0022] Get 40 Wistar male rats, remove the hair on the back 24 hours before the experiment, perform aseptic operation under light ether anesthesia, inject 2% agar solution 2ml / rat subcutaneously in the hair-removed area of the back midline of the rats to cause inflammation, and from the day of inflammation, divide 4 groups were given intragastric administration. The compound of Formula 1 was divided into high concentration group and low concentration group. The administration concentration is shown in Table 1. The administration was once a day for a total of 14 days. On the 15th day, the rats were sacrificed, dissected, the granuloma agar block was peeled off, and the wet weight was weighed.
[0023] The influence of table 2 formu...
Embodiment 310
[0026] Example 310-O-(Dimethylaminoethyl) ginkgolide B is on the effect of carrageenan-induced joint swelling in rats
[0027] 3.1 Grouping and administration
[0028] Get 36 rats, be divided into 6 groups at random, be respectively: (1) blank control group: NS 10ml / kg; (2) dexamethasone group: 5mg / kg; (3) formula 1 compound I group: 20mg / kg kg; (4) Formula 1 compound II group: 40 mg / kg; (5) Formula 1 compound III group: 80 mg / kg; (6) Formula 1 compound IV group: 160 mg / kg. Rats in each group were intragastrically administered with the above-mentioned dose of 10ml / kg, once a day, for 5 consecutive days.
[0029] 3.2 Measuring indicators and methods
[0030] One hour after the last administration, subcutaneously inject 0.1% carrageenan solution 0.1ml / paw into the sole of the left hind foot to cause inflammation. The volume of the toe of the left hind paw of the mouse.
[0031] Record the results, and calculate the swelling rate (%) and inhibition rate (%) respectively accor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com