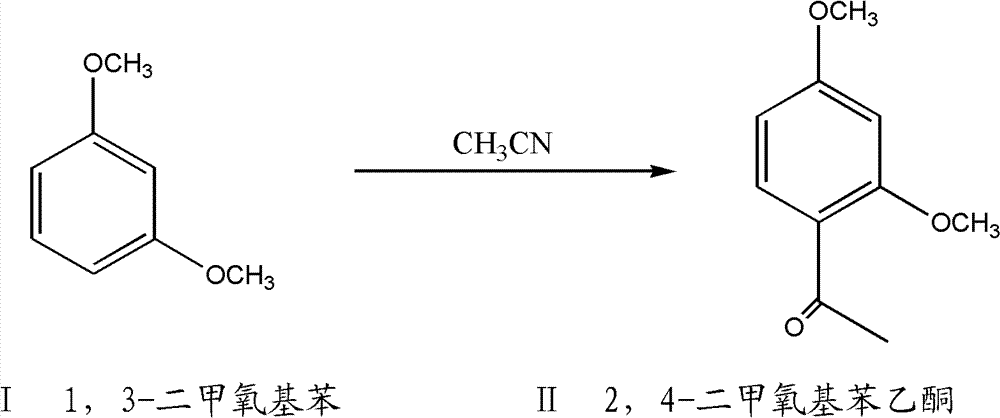
A method for preparing 2,4-dimethoxyacetophenone
A technology of methoxyacetophenone and dimethoxybenzene, which is applied in the field of chemical intermediates 2, can solve the problems of dangerous large trifluoromethanesulfonic acid and low product yield, and achieve easy industrial implementation and high reaction yield. The effect of high yield and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] 138.2g (1.0mol) of 1,3-dimethoxybenzene and 415.0g of toluene were added to a 1000ml reaction flask, the temperature was lowered to -10°C, 133.3g of aluminum trichloride was added under stirring, and 82.1g of acetonitrile was added dropwise. Pass through dry hydrogen chloride gas, keep it warm at -5~5°C for 20 hours, stop stirring, and filter to obtain a solid; add 300.0 g of water to heat and reflux for 1 hour, cool down and filter to obtain 2,4-dimethoxybenzene Ethyl ketone weighed dry to be 165.8g, the yield was 92.0%, and the content was 99.0%.
Embodiment 2
[0031] 138.2g (1.0mol) of 1,3-dimethoxybenzene and 830.0g of toluene were added to a 2000ml reaction flask, the temperature was lowered to -10°C, 200.0g of aluminum trichloride was added under stirring, and 120.0g of acetonitrile was added dropwise. Pass through dry hydrogen chloride gas, keep it warm at -10~0°C for 30 hours, stop stirring after the reaction, and filter to obtain a solid; add 300.0 g of water to heat and reflux for 1 hour, cool down and filter to obtain 2,4-dimethoxybenzene Ethyl ketone weighed dry to be 168.4g, the yield was 93.4%, and the content was 99.5%.
Embodiment 3
[0033] 138.2g (1.0mol) of 1,3-dimethoxybenzene and 1000.0g of toluene were added to a 2000ml reaction flask, the temperature was lowered to -10°C, 220.0g of aluminum trichloride was added under stirring, and 110.0g of acetonitrile was added dropwise. Pass through dry hydrogen chloride gas, keep it warm at 5-15°C for 10 hours, stop stirring after the reaction, and filter to obtain a solid; add 300.0g of water to heat and reflux for 1 hour, cool down and filter to obtain 2,4-dimethoxyphenylethyl The ketone weighed dry was 166.4g, the yield was 92.3%, and the content was 98.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com