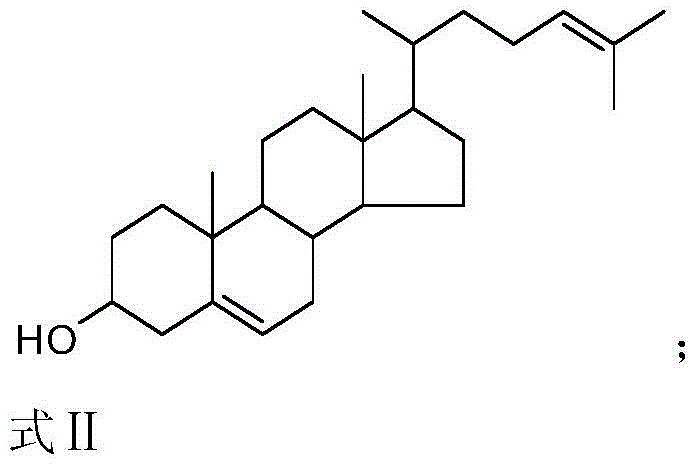
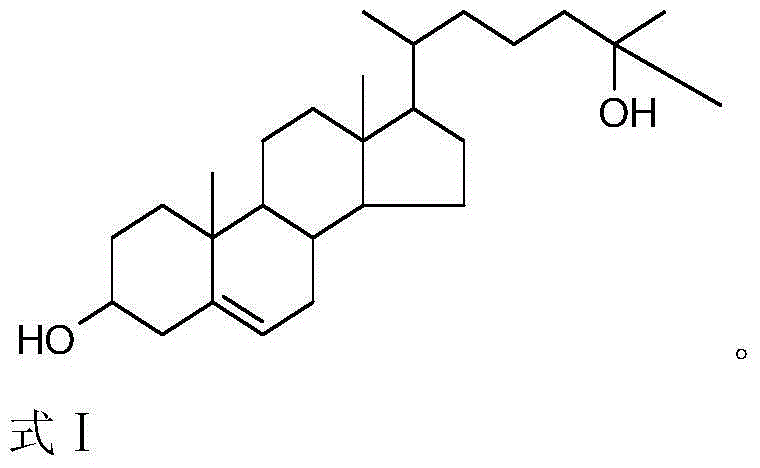
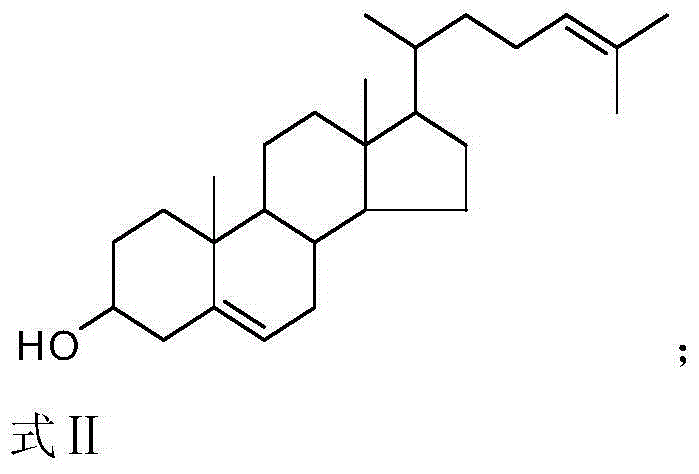
Synthetic method of 25-hydroxycholesterol
A technology of hydroxycholesterol and dehydrocholesterol, which is applied in the field of preparation of 25-hydroxycholesterol, can solve the problems of poor selectivity and low yield, and achieve the effects of high selectivity, high yield and environmental friendliness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1. A method for preparing 25-hydroxycholesterol, the following steps are carried out in sequence:
[0048] 1) Use 120mL pyridine as solvent, 30mL pyridine (about 0.372mol) as acid binding agent, add 20g 24-dehydrocholesterol (about 0.052mol), 0.2g 4-dimethylaminopyridine (DMAP), drop at room temperature Add 10g (about 0.097mmol) of acetic anhydride (dropped in about 30 minutes). The reaction process was monitored by TLC. After the addition, the reaction was continued at room temperature for 10 hours, and the reaction was completed.
[0049] The reaction liquid is washed with water (50ml×2 water), acid washed (with a volume concentration of 5% dilute hydrochloric acid solution, 50ml×2) and then extracted with dichloromethane (30ml×3), and the extract is used The saturated sodium bicarbonate solution was washed to neutrality, anhydrous sodium sulfate (about 5g) was dried and the solvent (ie, dichloromethane) was removed to obtain 19.1g of acetylated 24-dehydrocholeste...
Embodiment 2
[0056] Example 2. A method for preparing 25-hydroxycholesterol,
[0057] In this embodiment 2, steps 1) and 2) are different from the embodiment 1. The difference lies in:
[0058] 1) Using 100mL of dichloromethane as a solvent, add 20g of 24-dehydrocholesterol (about 0.052mol), 0.2g of DMAP, 10.1g (about 0.1mmol) of triethylamine, and dropwise add 10g (about 0.097mmol) of vinegar at room temperature anhydride. The reaction process was monitored by TLC. After the addition, the reaction was continued at room temperature for 10 hours, and the reaction was completed.
[0059] The reaction solution was washed with dilute hydrochloric acid (volume concentration 5%, 50ml×2), saturated sodium bicarbonate solution (30ml×2), saturated sodium chloride solution (30ml) and dried with anhydrous sodium sulfate (about 5g). The solvent (ie, dichloromethane) was removed by rotary evaporation under reduced pressure (0.1MPa) to obtain 19.0 g of acetylated 24-dehydrocholesterol with a yield of 90.0%. ...
Embodiment 3
[0061] Example 3. A method for preparing 25-hydroxycholesterol,
[0062] In this embodiment 3, steps 1) and 2) are different from the embodiment 1. The difference lies in:
[0063] 1) Using 100mL of dichloromethane as solvent, add 20g of 24-dehydrocholesterol (about 0.052mol), 0.2g of DMAP, 10.1g (about 0.1mmol) of triethylamine, and add 14.1g (about 0.1mol) dropwise at room temperature After the addition of benzoyl chloride, the temperature was raised to reflux (about 40°C), the reaction process was monitored by TLC, and the reaction was completed after refluxing for 24 hours.
[0064] The reaction solution was washed successively with dilute hydrochloric acid, saturated sodium bicarbonate solution, saturated sodium chloride solution and then dried over anhydrous sodium sulfate, and the solvent was removed by rotary evaporation under reduced pressure to obtain 23.2 g of benzoylated 24-dehydrocholesterol. The yield was 95.0%.
[0065] 2) Change 0.213g (1.2mmol) of N-bromosuccinimide ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com