Improved culture medium for neonatal pig islet cells and use method of same
A technology of islet cells and culture medium, which is applied in the field of improved newborn pig islet cell culture medium, which can solve the problems of unfavorable treatment effect, decreased insulin secretion, difficult to remove pollution, etc., to increase the recovery rate of cultured cells and increase the secretion of insulin , the effect of reducing the number of apoptotic cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
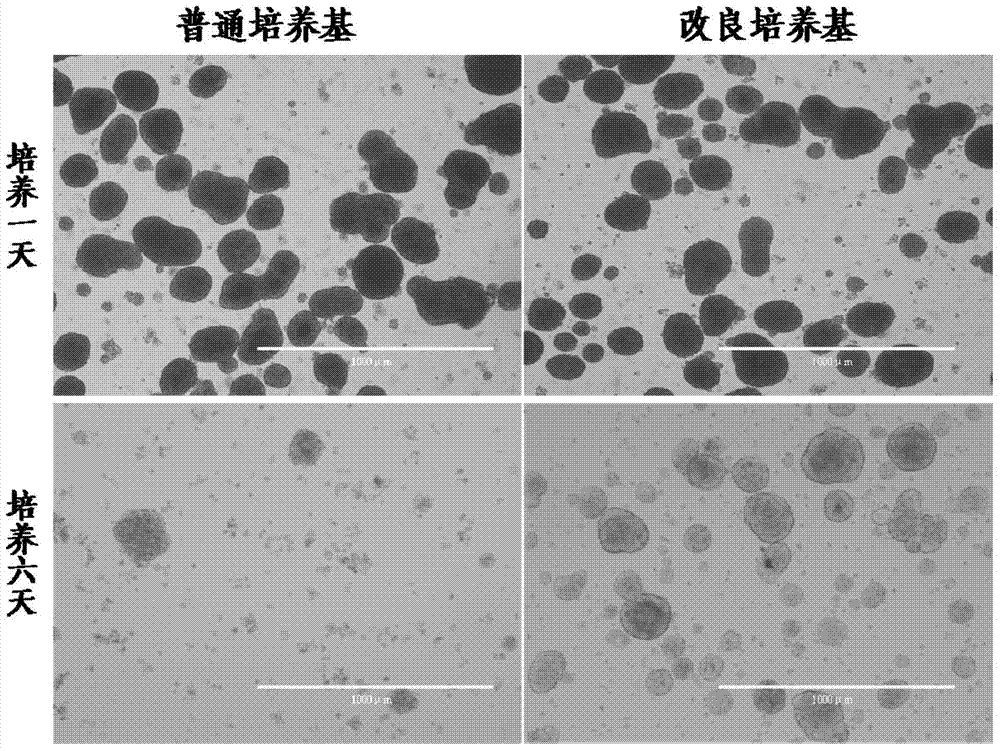
[0032] Embodiment 1, culture medium of the present invention and culture method test
[0033] The present invention injects 10IU / kg heparin sodium intraperitoneally before taking pancreas from newborn pigs. The inoculation concentration of the present invention is 5000-10000IEQ / 25-30ml for isolated and purified newborn pig islet cells in the culture medium, culture medium (HAM'S F10 Base and added with apoptosis inhibitor Z-VAD-FMK20μΜ, human basic fibroblast growth factor hFGF-β10-20μg / L, vascular endothelial growth factor VEGF10-20μg / L, insulin-like growth factor R3-IGF-110 -20μg / L, human epidermal growth factor hEGF10-20μg / L, hepatocyte growth factor hHGF10-20μg / L and pig serum accounting for 10% of the medium volume, as well as sodium selenite 6.7ng / L, insulin 10μg / L L, transferrin 5.5μg / L, ethanolamine 2μg / L, 1-methyl 3-isobutylxanthine 0.011g / L, nicotine 1.22g / L, cortisol (hydrocortisone) 5μM, 80U / ml penicillin, 100U / ml streptomycin) at 37°C, 5% CO 2 , Cultured in a 95...
Embodiment 2
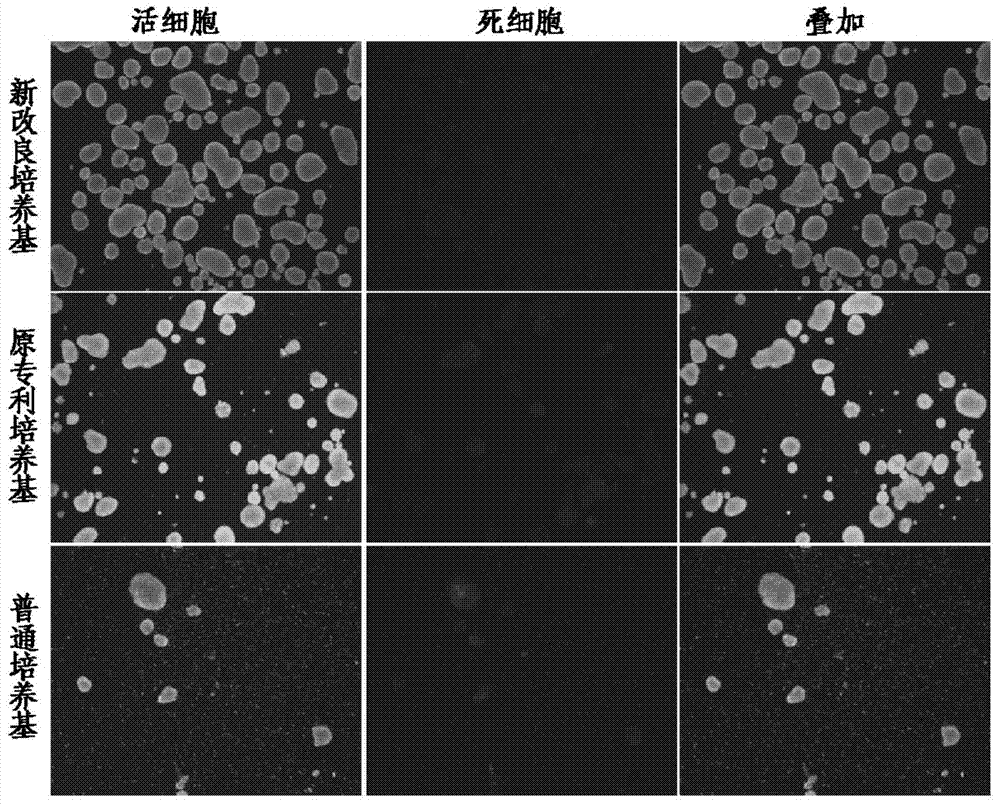
[0040] Example 2, the effect comparison between the present invention and patent application 201210330427.9, and general cultivation methods, the results are shown in Table 1-3.
[0041] The survival rate of active islet cells cultured in the present invention for 6 days reaches 97.68±1.75%, which is higher than that of general cultured islet cells (<70%) and the original patent 201210330427.9 (≤82%); the survival rate of active pancreatic islet cells cultivated in the present invention for 10 days reaches 88.9% ±1.63%, much higher than the general medium (<10%) and the original patent 201210330427.9 (69.20%); the percentage of β cells cultured for 6 days in the present invention is 85.25±1.69%, which is also higher than the general medium (<50%) and the original patent 201210330427.9 (≤72.0%); the percentage of β cells cultured for 10 days in the present invention reaches 96.8±0.45%, which is higher than that of ordinary culture medium (<60%) and the original patent 2012103304...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com