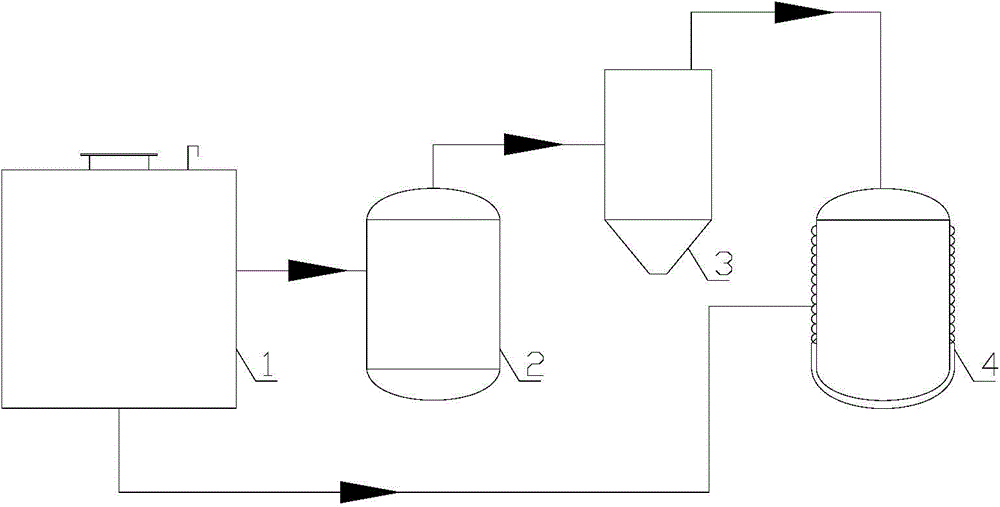
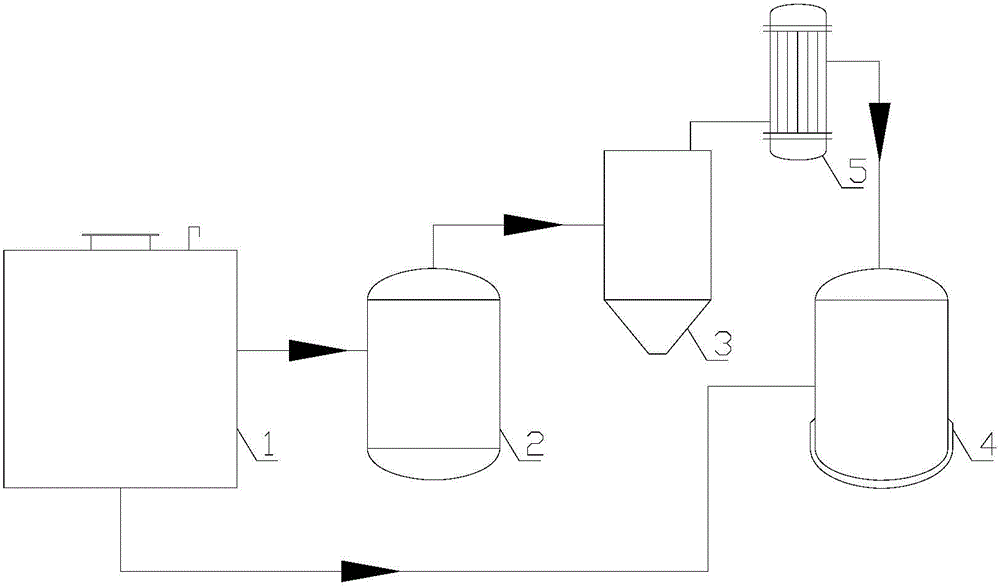
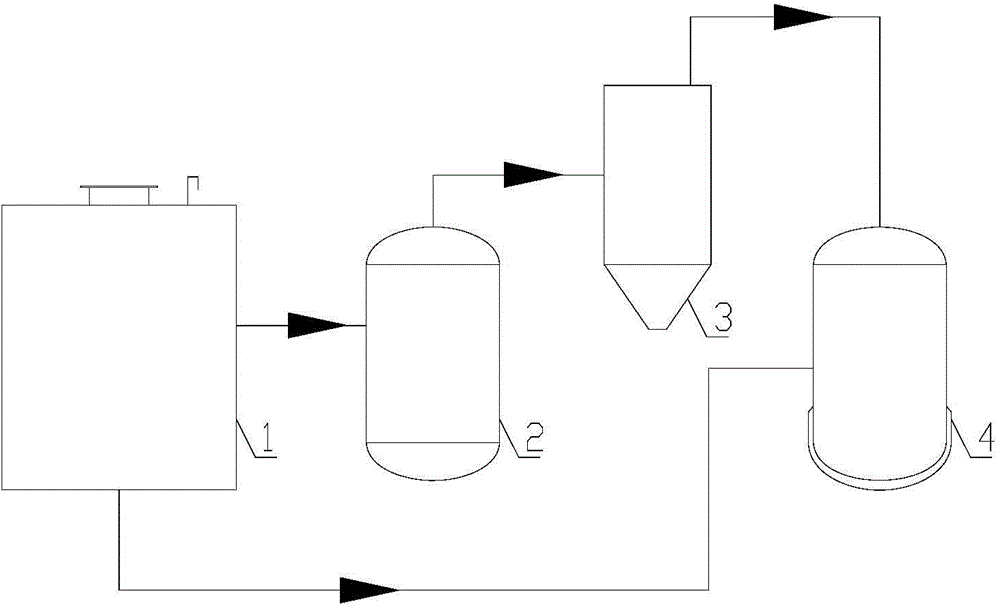Method and equipment for recycling fluoride in iron-containing compound production
A technology of fluoride and compounds, which is applied in the field of fluoride recycling in the production of iron-containing compounds, can solve the problems of polluting the environment and reducing the utilization rate of fluorine-containing compounds, and achieve the effect of reducing repeated recycling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] will contain Fe 2 o 3 waste with CaF 2 Mix well to get a mixture, Fe 2 o 3 with CaF 2 The particle size is below 3mm, the CaF in the resulting mixture 2 The mass percentage is 80%. Calcinate the mixture in the first reactor at 1150°C for 1 hour to generate CaO and FeF 3 , FeF 3 It is gaseous at high temperature and escapes from solid reactants. FeF that will escape 3 Introduced into the second reactor and hydrolyzed at a temperature of 1250°C to generate Fe 2 o 3 and HF gas. The HF gas produced by hydrolysis is transported to the third reactor with a polytetrafluoroethylene pipeline, and reacted with the reaction product CaO at 600°C for 2.5 hours to obtain CaF again. 2 , thus realizing CaF 2 of recycling. The reaction equation is as follows:
[0066] Fe 2 o 3 +3CaF 2 →3CaO+2FeF 3 (4)
[0067] 2FeF 3 +3H 2 O → Fe 2 o 3 +6HF (5)
[0068] CaO+2HF→CaF 2 +H 2 O (6)
Embodiment 2
[0070] Magnetite (Fe 2 o 3 mSiO 2 ) with AlF 3 Mix well to get a mixture, magnetite and AlF 3 The particle size is below 5mm, and the AlF in the resulting mixture 3 The mass percentage is 60%. The mixture was calcined at 1300°C for 3 hours in the first reactor to generate Al 2 o 3 and gaseous FeF 3 and gaseous SiF 4 , the gaseous SiF 4 After being separated at low temperature, it is hydrolyzed to produce silicon dioxide and hydrogen fluoride. FeF 3 Escape from the solid reactant by sublimation. FeF that will escape 3 Introduced into the second reactor and hydrolyzed at 1300°C to generate Fe 2 o 3 and HF gas. The HF gas produced by hydrolysis is transported to the third reactor with polytetrafluoroethylene pipeline, and the reaction product Al 2 o 3 After reacting at 100°C for 5 hours, AlF was recovered 3 , thus achieving AlF 3 of recycling. The reaction equation is as follows:
[0071] Fe 2 o 3 +2AlF 3 →Al 2 o 3 +2FeF 3 (7)
[0072] 3SiO 2 +4A...
Embodiment 3
[0076] Will Fe 2 o 3 Coarse particles and NH 4 HF 2 Mix well, Fe 2 o 3 with NH 4 HF 2 The particle size is below 7mm, and the NH in the resulting mixture 4 HF 2 The mass percentage is 40%. The mixture was calcined at 600°C for 5 hours in the first reactor to generate NH 3 、H 2 O and FeF 3 , FeF 3 escaped from the solid. FeF that will escape 3 Introduced into the second reactor and hydrolyzed at 1100°C to generate Fe 2 o 3 and HF gas. The HF gas produced by hydrolysis is transported to the third reactor with polytetrafluoroethylene pipeline, and the reaction product NH 3 and H 2 O was reacted at 150°C for 4.5 hours to regain NH 4 HF 2 , thus achieving NH 4 HF 2 of recycling. The reaction equation is as follows:
[0077] Fe 2 o 3 +3NH 4 HF 2 →2FeF 3 +3NH 3 +3H 2 O (11)
[0078] FeF 3 +3H 2 O → Fe 2 o 3 +6HF (12)
[0079] NH 3 ·H 2 O+2HF→NH 4 HF 2 +H 2 O (13)
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com