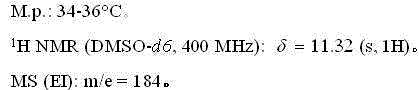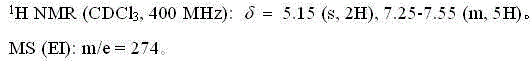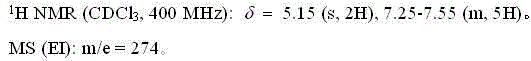Method for synthesizing 2,3,4,5,6-pentafluorophenol
A technology of pentafluorophenol and pentafluorobromobenzene, which is applied in the field of compound preparation, can solve the problems of inconvenient operation, difficult preparation, and low yield, and achieve the effects of reducing the use of expensive reagents, facilitating industrial production, and reducing costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Preparation of compound (II), namely benzyl-protected pentafluorophenol.
[0025] At room temperature, 95 mg of cuprous iodide, 0.15 g of 8-hydroxyquinoline, 4.24 g of potassium phosphate, and N 2 Under protection, add 1.25ml of pentafluorobromobenzene and 10ml of benzyl alcohol of the formula (I) with a syringe, slowly heat to 100°C, and react for 24 hours. After the reaction, cool down to room temperature. The reaction solution was diluted with dichloromethane, filtered, the filter cake was washed with dichloromethane, and the solvent was evaporated to obtain 2.2 g of compound (II), with a yield of 80%.
[0026] The NMR and mass spectrum data of compound (II) are as follows:
[0027]
Embodiment 2
[0029] Preparation of compound (II), namely benzyl-protected pentafluorophenol.
[0030] At room temperature, 95 mg of cuprous iodide, 0.15 g of 8-hydroxyquinoline, 4.24 g of potassium phosphate, and N 2 Under protection, add 1.25ml of pentafluorobromobenzene of formula (I) and 10ml of benzyl alcohol with a syringe, slowly heat to 110°C, and react for 36 hours. After the reaction, cool down to room temperature. The reaction solution was diluted with dichloromethane, filtered, the filter cake was washed with dichloromethane, and the solvent was evaporated to obtain 2.24 g of compound (II), with a yield of 82%.
[0031] The NMR and mass spectrum data of compound (II) are as follows:
[0032]
Embodiment 3
[0034] Preparation of compound (II), namely benzyl-protected pentafluorophenol.
[0035] At room temperature, 95 mg of cuprous iodide, 0.15 g of 8-hydroxyquinoline, 4.24 g of potassium phosphate, and N 2 Under protection, add 1.25ml of pentafluorobromobenzene of formula (I) and 10ml of benzyl alcohol with a syringe, slowly heat to 120°C, and react for 24h. After the reaction, cool down to room temperature. The reaction solution was diluted with dichloromethane, filtered, the filter cake was washed with dichloromethane, and the solvent was evaporated to obtain 2.16 g of compound (II), with a yield of 79%.
[0036] The NMR and mass spectrum data of compound (II) are as follows:
[0037]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com