Diterpene dimer compounds and pharmaceutical compositions and preparation method and application thereof
A technology of diterpene dimers and compounds, applied in the field of natural medicinal chemistry, can solve the problems of few research reports and achieve good anti-tumor activity and good anti-parasitic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
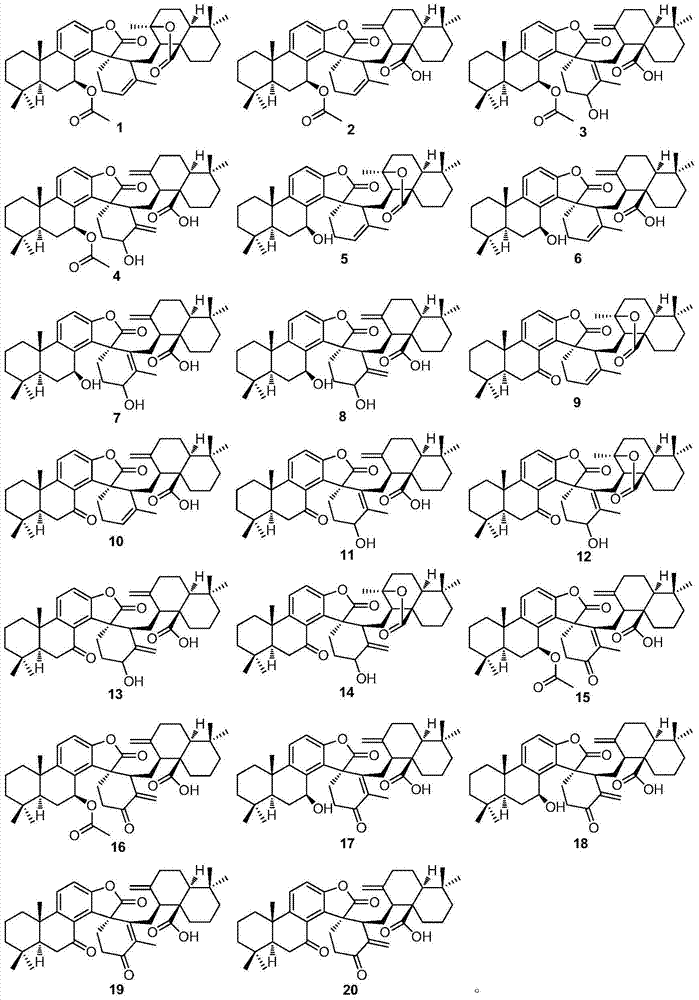
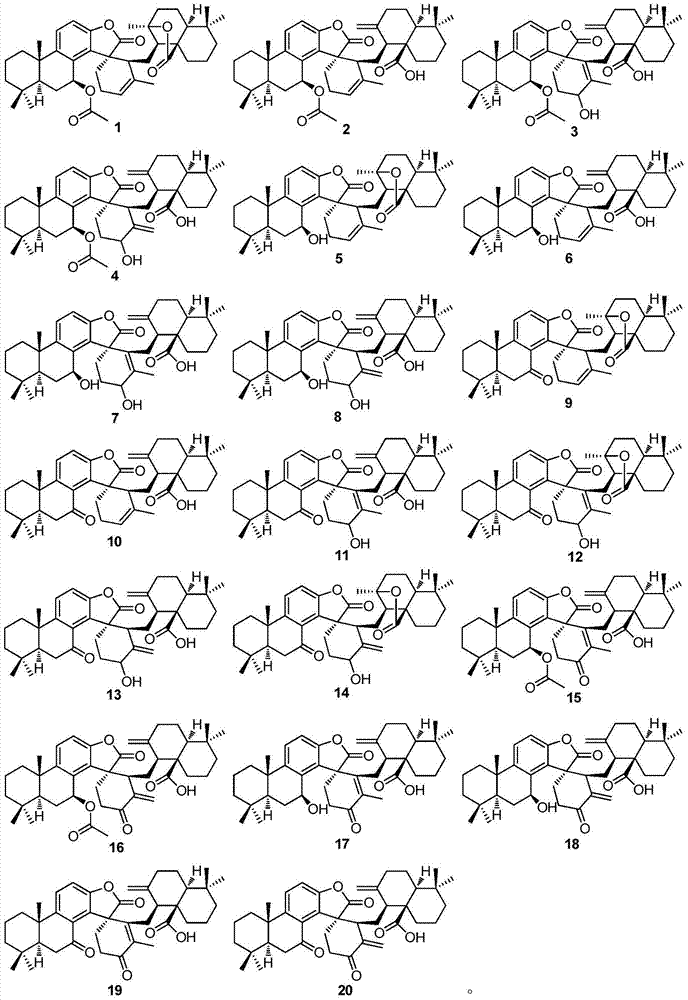
[0029] Extraction, separation and purification of compound 1-20 of the present invention:
[0030] Dry the rhizomes (5.5kg) of the genus Camellia in the shade, crush them to 30 meshes, extract them three times with 70% acetone at room temperature, 25 L each time, 24 hours, combine the extracts, concentrate the extracts under reduced pressure to obtain the extract Suspend with appropriate amount of water, then distribute with ethyl acetate several times to obtain ethyl acetate extract (115g), dissolve the extract with appropriate amount of chloroform / acetone, mix the sample with silica gel 80-100 mesh, and then use 1.2kg silica gel 200 -300 mesh column chromatography for rough separation, gradient elution with chloroform / acetone (1:0-0:1) to obtain 8 main fractions, the chloroform fraction and 9:1 chlorine / acetone fraction were subjected to silica gel column Chromatography, gradient elution with 200:1-2:1 petroleum ether / ethyl acetate, yielded 10 fractions. Compounds 1, 3 and ...
Embodiment 2
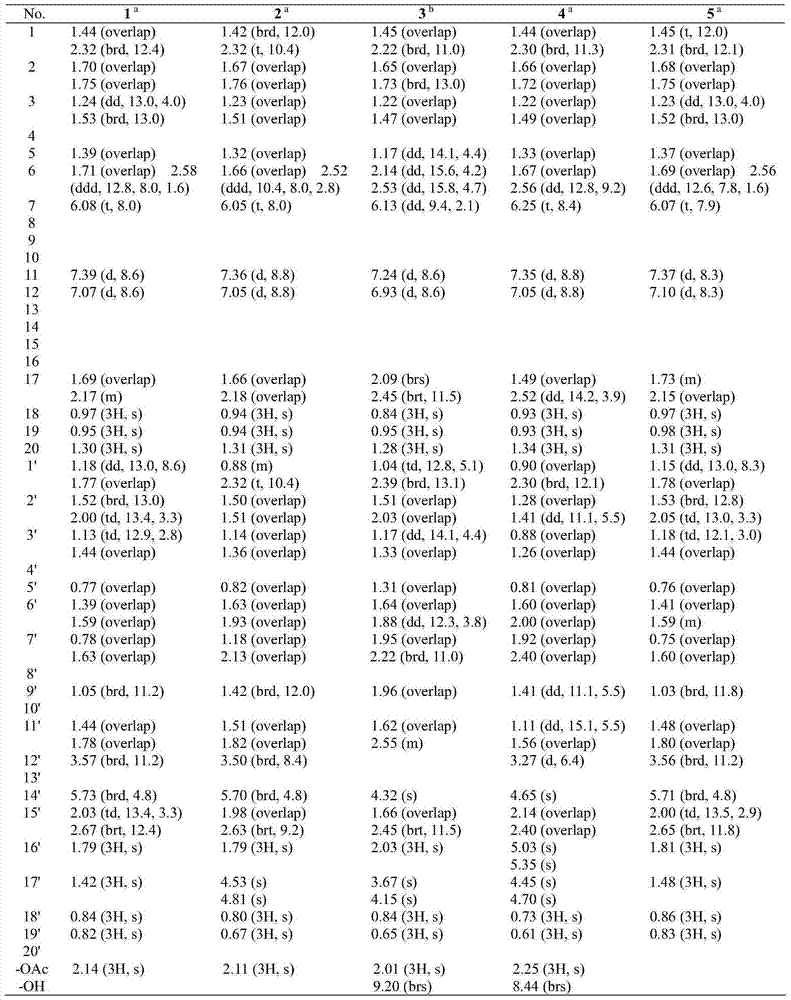
[0032] Physical and Spectroscopic Data of Compounds 1-20 of the Invention:
[0033] Compound 1: colorless crystals (acetone), UV(MeOH)λ max (logε)289.4(3.45),209.6(4.63)nm; IR(KBr)v max 3020,2928,2868,1800,1762,1728,1634,1610,1586,1458,1373,1278,1218,1132,1027,943,823,756cm -1 ;EIMS m / z656[M] + (5), 596(25), 294(44), 85(75), 83(100), 69(40); 1 H and 13 See Table 1 and Table 2 for C NMR data.
[0034] Compound 2: colorless crystals (acetone), UV(MeOH)λ max (logε)285.6(3.45),208.6(4.58)nm; IR(KBr)v max 3432,3085,2962,2929,2867,2847,1803,1738,1700,16471612,1587,1461,1368,1226,1127,1028,985,942,885,816cm -1 ;ESIMS m / z1336[2M+Na+H] + ,680[M+Na+H] + ,619[M+Na–HOAc] + ,551[M–HOAc–COOH] + ; 1 H and 13 See Table 1 and Table 2 for C NMR data.
[0035] Compound 3: colorless crystals (acetone), UV(MeOH)λ max (logε)284.1(3.73),204.0(4.90)nm; IR(KBr)v max3421,3081,2930,2869,1800,1737,1716,1647,1466,1368,1231,1125,1045,945,819,756cm -1 ;EIMS m / z672[M] + (4),671(8),628...
Embodiment 3
[0085] Cytotoxic activity detection of compounds of the present invention:
[0086] The cytotoxicity of the compound of the present invention to human gastric cancer cell line (SGC-7901), liver cancer cell line (SMMC-7721) and human erythrocytic leukemia cell line (K-562) was determined by MTT method. In the experiment, a negative control group (water), a DMSO solvent control group, a positive control group (mitomycin C) and different concentrations of test samples were set up, and each concentration was set in 2 parallels. Cells in the logarithmic growth phase were collected, counted with a hemocytometer, and inoculated in a 96-well flat-bottomed cell culture plate according to the amount of 4500 cancer cells per well, placed in 5% CO 2 , Humidity above 90%, cultivated in a 37°C incubator. After 24 hours, take out and add a certain amount of samples to be tested, continue to cultivate for 3 days, take out and place under a microscope to observe the cell morphology of each we...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com