Method for preparing high-stability asymmetric dimethylarginine hydrolase
A dimethylarginine, high-stability technology, applied in the field of protein purification, can solve the problems of unsatisfactory storage at room temperature, low purity, poor stability, etc., to achieve clinical use and storage, prolong storage time, and stabilize sex enhancing effect
Inactive Publication Date: 2014-06-11
浙江泰司特生物技术有限公司 +1
View PDF0 Cites 0 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
The expression and purification of DDAH are mostly used as experimental materials for functional research and drug screening models, so the production of DDAH is still limited to the laboratory level of mechanism research
Most of the produced
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
 Login to View More
Login to View More PUM
 Login to View More
Login to View More Abstract
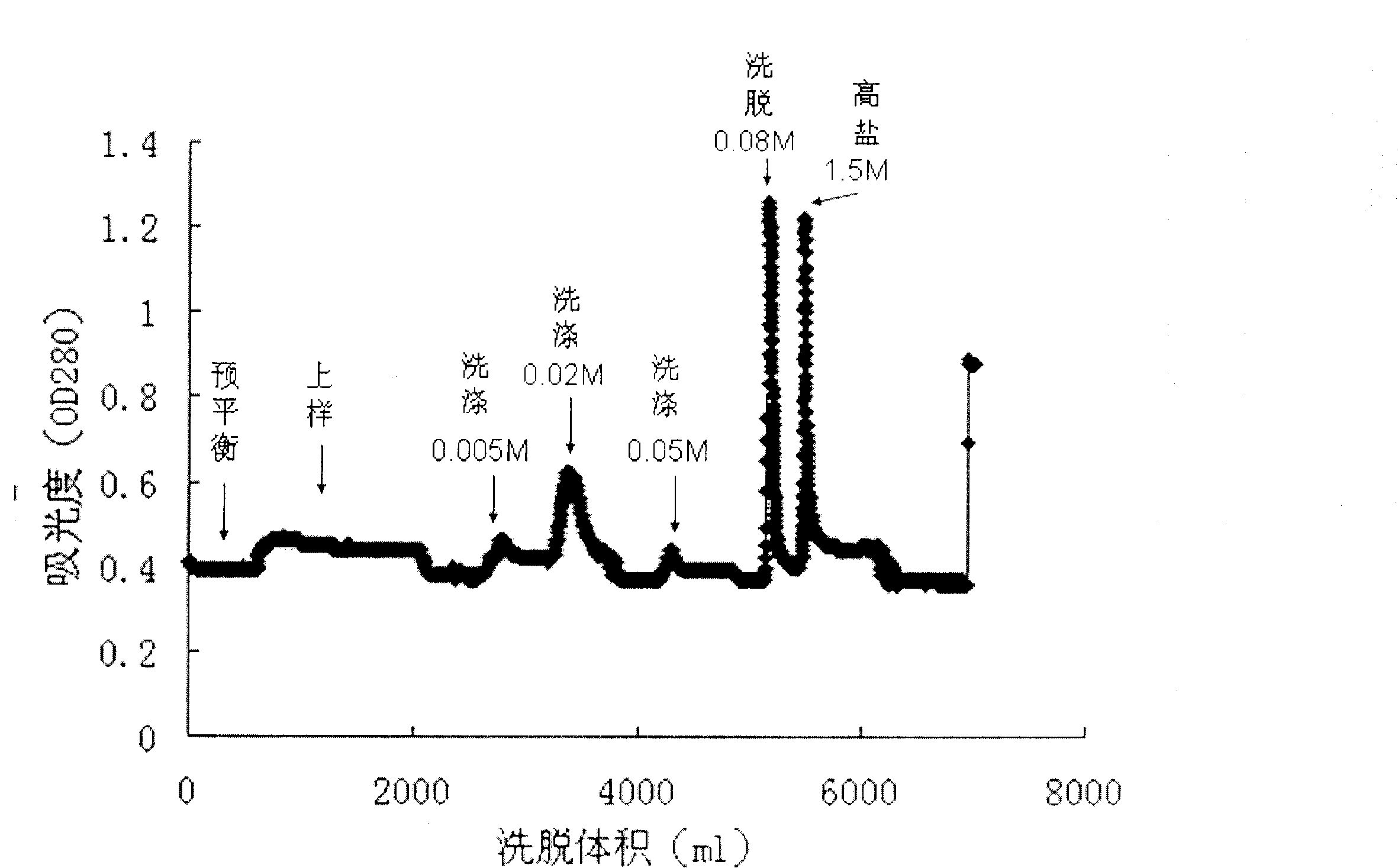
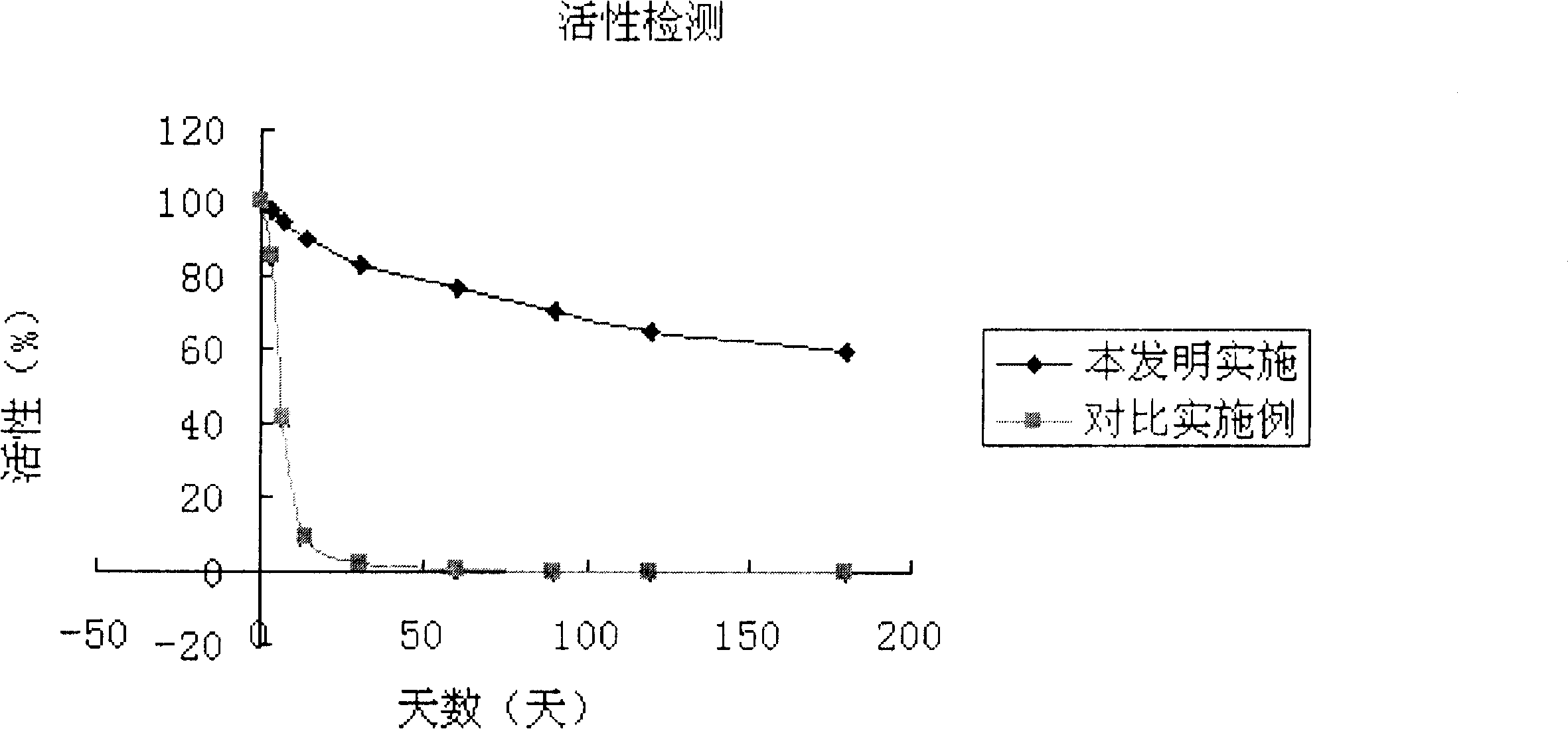
The invention belongs to the technical field of protein purification, in particular to a method for preparing high-stability asymmetric dimethylarginine hydrolase. The method comprises the following steps: loading asymmetric dimethylarginine hydrolase into an anion exchange chromatographic column; injecting at least one type of trihydroxymethyl aminomethane buffer solution into the column, wherein the anion concentration of the buffer solution is higher than the chromatographic column; removing protein impurities and eluting purified asymmetric dimethylarginine hydrolase from the column. In the embodiment, buffer solutions of four ion concentrations being approximately between 0mM and 500mM are adopted, so that gradient ion concentration is formed, impurities are removed, and high-purity asymmetric dimethylarginine hydrolase which is stable for a long time at the normal temperature is obtained.
Description
technical field [0001] The invention belongs to the technical field of protein purification, in particular to a method for preparing highly stable asymmetric dimethylarginine hydrolase. Background technique [0002] Asymmetric dimethylarginine (ADMA) is a biomarker of cardiovascular disease. The compound is produced intracellularly and then transferred to the plasma, where it inhibits the production of nitric oxide from arginine, a substance that is beneficial to endothelial cells and the cardiovascular system. Studies have shown that ADMA is a risk factor for critical illness, and its expression level in vivo has important predictive value and clinical significance for the occurrence, development and prognosis of acute myocardial infarction, atherosclerosis, diabetes and liver and kidney failure. Therefore, the establishment of a rapid and accurate detection method for ADMA content has very important significance for basic research and clinical diagnosis. [0003] At pres...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): C12N9/78
CPCC12N9/78C12Y305/03018
Inventor 王学忠胡广赵志敬
Owner 浙江泰司特生物技术有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com