Nonlinear polymer containing doxorubicin structure, and preparation method and application thereof
A doxorubicin and polymer technology, which can be used in medical preparations containing active ingredients, medical preparations without active ingredients, organic active ingredients, etc., can solve the problems of short half-life, low solubility, high toxicity, etc. Prolonged half-life, excellent effect, easy-to-dissolve effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
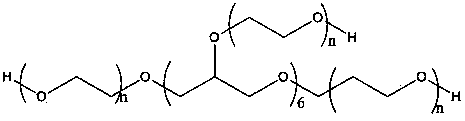
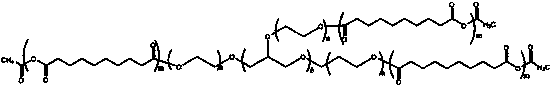
[0035] 1) The mixture of 80 g of sebacic acid in 800 ml of acetic anhydride is refluxed to form acetyl-sebacic acid;
[0036] 2) Mix the 18g product of step 1) and 8-arm polyethylene glycol ending in a hydroxyl group into a flask, and conduct a solution polymerization reaction under reduced pressure at 180°C for 1 hour; when the polymer is cooled to room temperature, dissolve it with chloroform, and use petroleum washed with ether and dried;
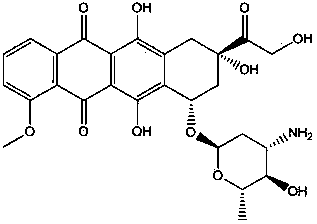
[0037] 3) Put 120mg of doxorubicin and the 800mg polymer of step 2 into 8ml of dimethyl sulfoxide and 12ml of dichloromethane solution for 48 hours; ultrasonic for 3 minutes; then place in an oven for 1 hour; Stir at a high speed in a homogenizer at 20°C for 3 minutes, then put into 1% polyvinyl alcohol solution and stir at 600 rpm for 2 hours; collect by centrifugation and freeze-dry to obtain the nanoparticles of the final product.
Embodiment 2
[0039] 1) The mixture of 100 g of sebacic acid in 900 ml of acetic anhydride is refluxed to form acetyl-sebacic acid;
[0040] 2) Mix the 30 g product of step 1) and 8-arm polyethylene glycol ending in a hydroxyl group into a flask, and conduct a solution polymerization reaction under reduced pressure at 180 ° C for 1 hour; when the polymer is cooled to room temperature, dissolve it with chloroform, and use petroleum washed with ether and dried;
[0041] 3) Put 200mg of doxorubicin and 1000mg of the polymer in step 2 into 6ml of dimethyl sulfoxide and 14ml of dichloromethane solution for 24 hours; ultrasonic for 2 minutes; then put in an oven for 2 hours; at minus 10 degrees Stir in a medium homogenizer at an ultra-high speed for 2 minutes, then put into 10% cholic acid solution and stir at 600 rpm for 3 hours; collect by centrifugation and freeze-dry to obtain the nanoparticles of the final product.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap