Brimonidine d-tartrate ophthalmic gel preparation as well as preparation method and application thereof
A technology of brimonil for eye fixation and ophthalmic gel, which is applied in the direction of non-active ingredient medical preparations, active ingredient-containing medical preparations, pharmaceutical formulas, etc., can solve problems such as limited effects, and achieve reduction of toxic and side effects, Prolong the residence time and solve the effect of rapid metabolism
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
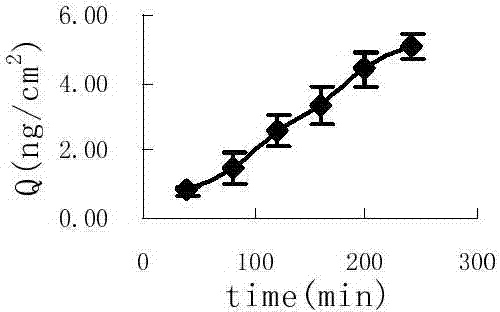
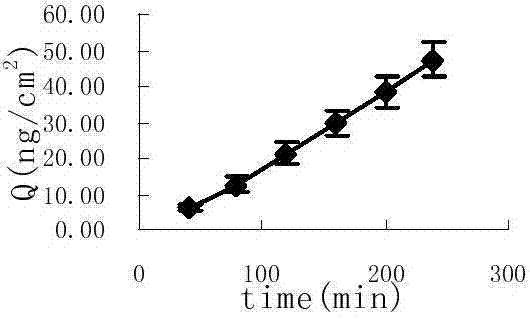
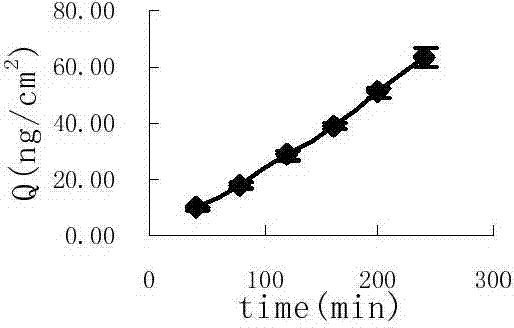
Image
Examples
Example Embodiment
Example 1
A brimonidine tartrate ophthalmic gel preparation was prepared, with a total mass of 100 g, of which, brimonidine tartrate was 0.01 g, 974P NF was 0.1 g, and the rest was water for injection.
The preparation method is as follows: (1) Prepare the gel matrix, add 0.1g 974P NF to 15g phosphate buffer under constant stirring, and stir evenly to obtain the gel matrix;
(2) Prepare a drug solution, gradually add 0.01 g of brimonidine tartrate to 5 g of water for injection, mix well, and prepare a drug solution;
(3) Mixing: The drug solution prepared in step (2) is added to the gel matrix prepared in step (1) under constant stirring, and the solution is filtered through a microporous membrane, and then injected from the membrane Use water to 100g.
Example Embodiment
Example 2-10
According to the method described in Example 1, the ophthalmic gel preparations in Examples 2 to 10 were prepared using the components and their weight parts as shown in Table 1, respectively; wherein, when a thickener is used, it is prepared The gel matrix is added to the buffer together with the gel material in the step of gel matrix, and when isotonic regulators and preservatives are used, it is added to the water for injection together with brimonidine tartrate in the step of preparing the drug solution.
Table 1 Components and weight parts of ophthalmic gel preparations
Note: The thickeners HPMC E4M, HPMC F4M, HPMCK4M used in this example are products produced by Dow Chemical, and their trade names are Metoprolum. TM E4M, Madoxiu TM F4M, Midasiu TM K4M.
Testing and evaluation
Corneal retention time test
The inventors selected sodium fluorescein as the color-developing material, and took Example 1, Example 2, Example 3, Example 5, Example 7, and Example 10...
Example Embodiment
Example 1 170**±12 151**±8
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com