Galactose‑rhamnose galacturonate composition for treating nonalcoholic steatohepatitis and nonalcoholic fatty liver disease
A technology for lactobionate and steatohepatitis, which is applied in the field of chemistry and liver, and can solve the problems of non-existence of specific therapy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0138] Embodiment 1: the method for manufacturing galactose-rhamnose galacturonate compound
[0139] The following are merely illustrative examples of generating therapeutic polysaccharides and are not intended to limit the present invention. In this case, the galactose-rhamnose galacturonate produced has been labeled GR-MD-02 in this application.
[0140] Apple pectin USP HM (50 kg) was dissolved and heated to 35-85°C in water. Add 1M HCl or NaOH to adjust the pH of the solution to pH 5-7 and mix well. Mixing was continued for 2 hours at the 35-85°C set point. 1M NaOH or HCl was added to readjust the pH to between 5 and 7 as needed. The solution was cooled to 30°C. The pH was adjusted to between 5 and 7 at 30°C.
[0141] CuSO 4 Added to pH-adjusted pectin solution to form 1 mM final CuSO 4 concentration. 1mM CuSO 4 The solution was mixed for 30 minutes at a temperature between 10°C and 30°C.
[0142] 1 mM CuSO for 30 min 4 At the end of the mixing step, 50 grams ...
Embodiment 2
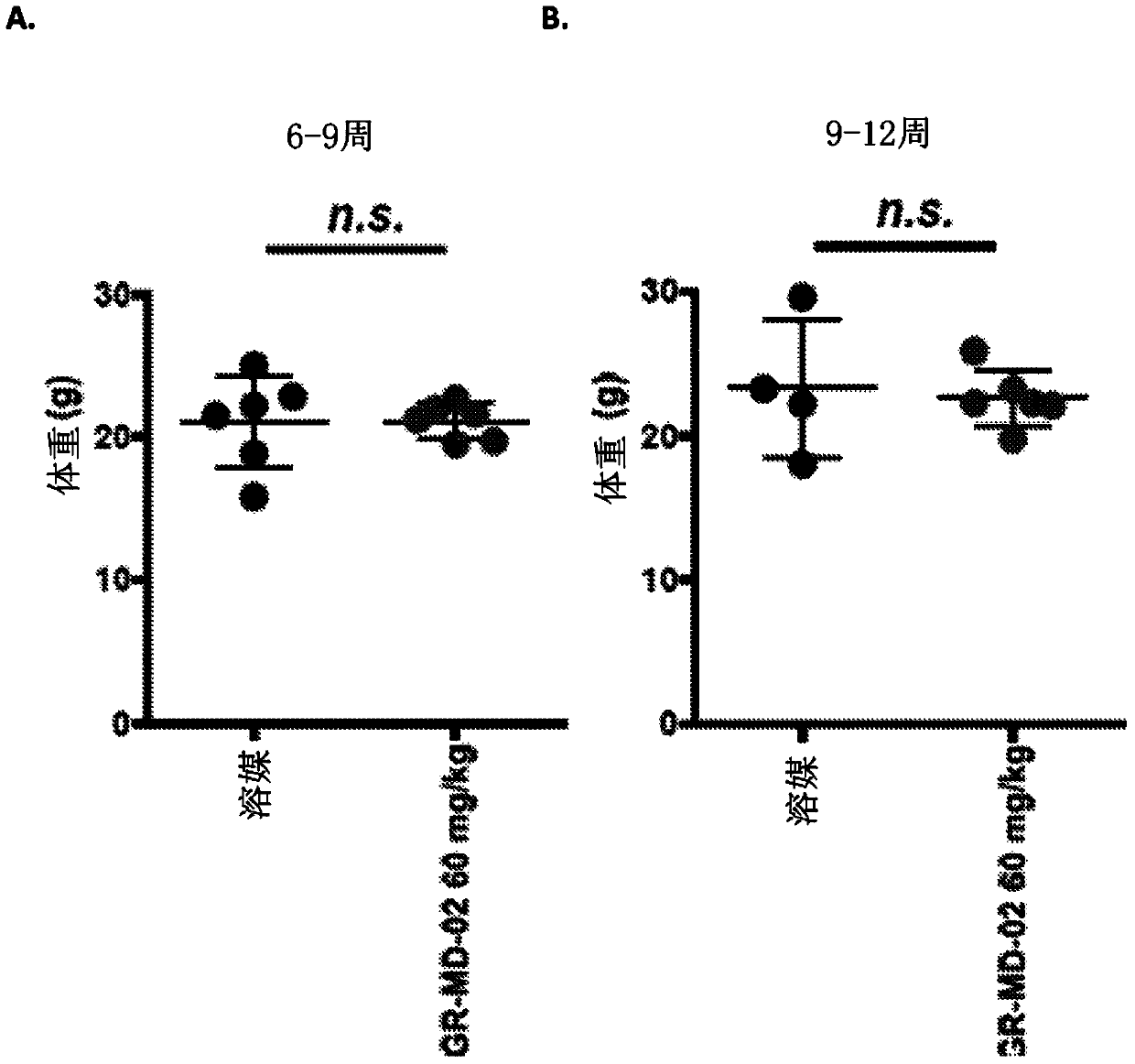
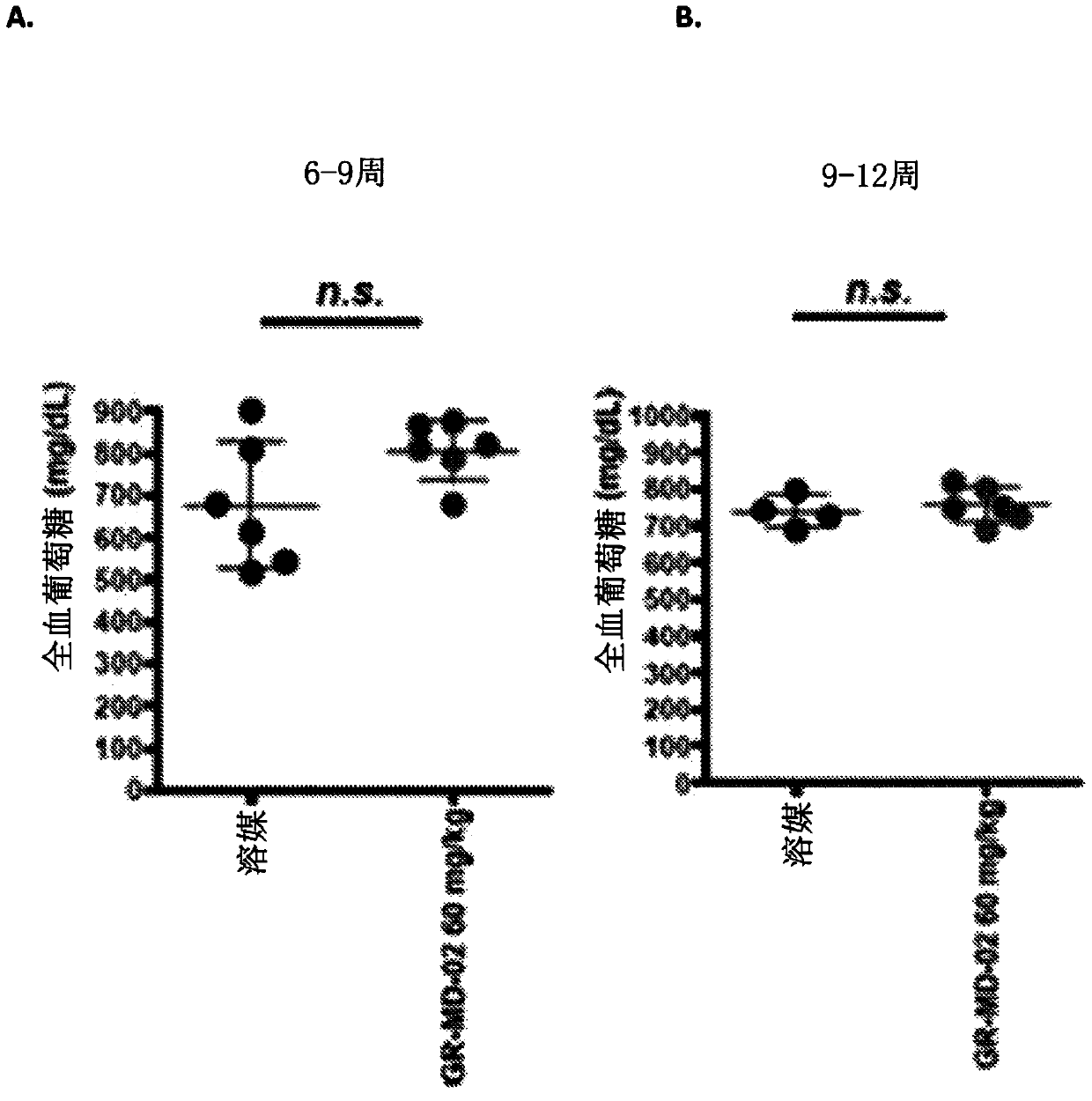
[0147] Embodiment 2: the method for treating steatohepatitis mouse model
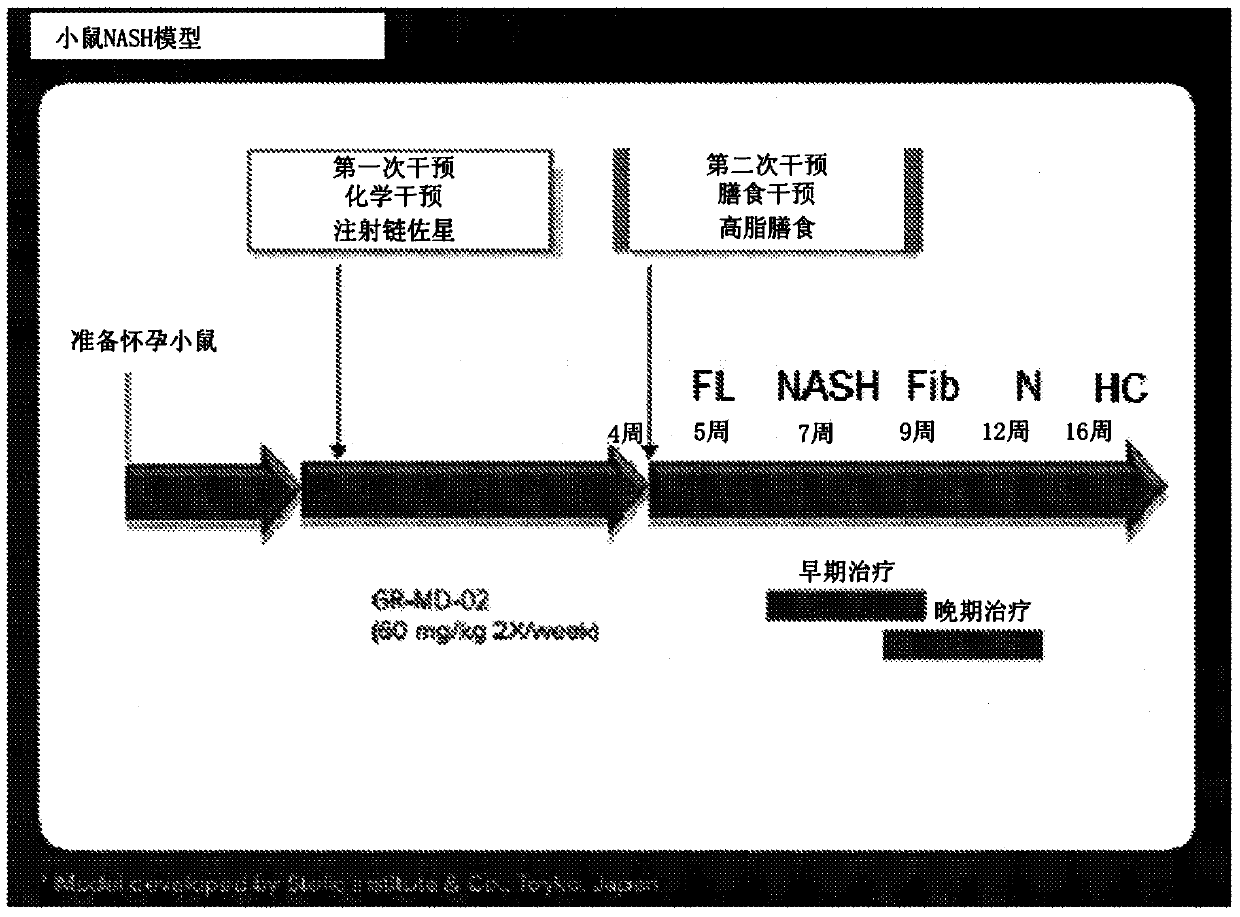
[0148] The experimental model used in this example was a mouse in which diabetes was induced and a high-fat diet was administered, a model that has been called STAM mouse. Such as figure 1Diabetes was induced with a single injection of streptozocin immediately after birth as shown in , and mice were then fed a high-fat diet 4 weeks later. This is a proven model in which mice consistently develop NASH with hepatocyte fat accumulation, signs of hepatotoxicity, portal and lobular inflammatory infiltrates, perisinusoidal fibrosis, advanced fibrosis with nodular formation, and cirrhosis And eventually hepatocellular carcinoma develops in a certain percentage of animals.
[0149] Disease progression was fatty liver (FL) by 5 weeks of age, steatohepatitis (NASH) by 7 weeks of age, fibrosis (Fib) by 9 weeks of age, nodules (N) by 13 weeks of age , and some animals developed hepatocellular carcinoma (HC) b...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com