Method for performing in-situ controllable coating on lithium ion battery electrode material by phenolic resin
A lithium-ion battery and electrode material technology, applied in battery electrodes, secondary batteries, circuits, etc., can solve the problems of uneven and continuous carbon layer and unsatisfactory coating effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0126] Embodiment 1, utilize method a to carry out carbon layer coating
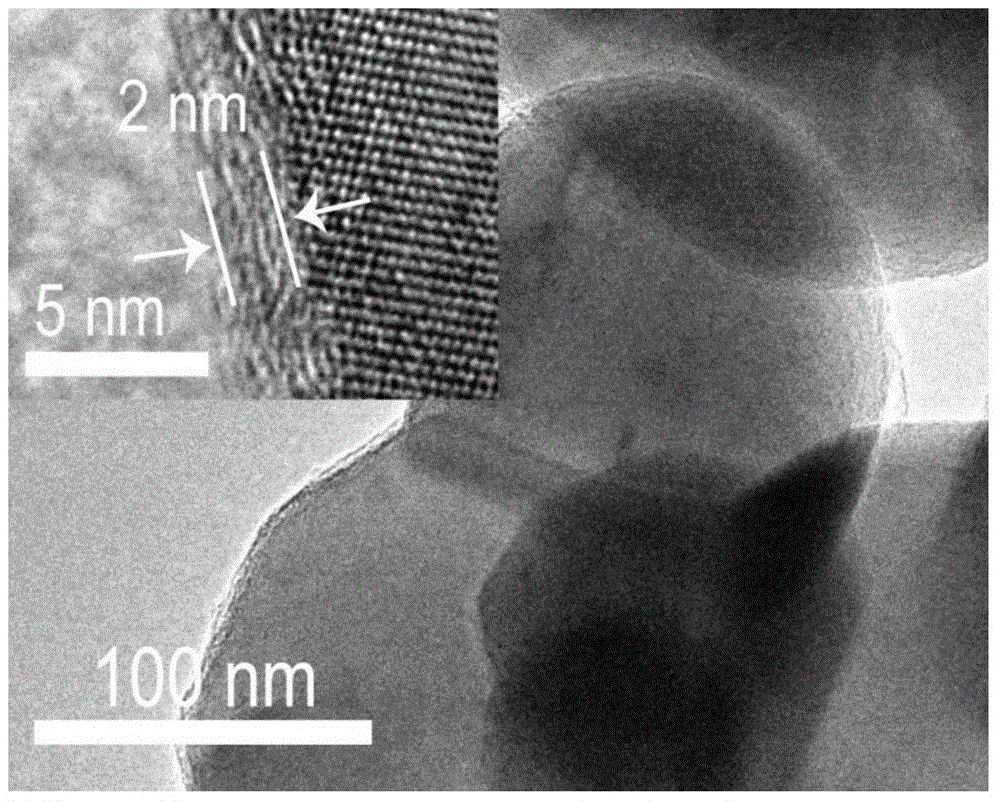
[0127] 1) Weigh 1.0g (6.34mmol) cathode material LiFePO with a particle size of 150nm 4 In the presence of 30ml H 2 In a mixed solution of O and 15ml EtOH, ultrasonically disperse for 15-30min. Then add 0.05g (0.455mmol) resorcinol solid powder successively, 0.2ml mass percent concentration is the ammoniacal liquor (1.30mmol) of 25% and 0.1ml mass concentration is the formaldehyde aqueous solution (1.34mmol) of 37%, at room temperature Stir for 24h. The precipitate was collected by centrifugation, washed three times with water and once with ethanol, and the obtained precipitate was fully dried in a drying oven at 80°C for 12 hours to obtain the intermediate product a;
[0128]2) Place the dried powder intermediate a (referred to as LFPRF) in a tube furnace with hydrogen-argon gas mixture (5 / 95% by volume), calcinate at 400°C for 4h, and then raise the temperature to 700°C ℃, calcined for 15h, and nat...
Embodiment 2
[0130] Embodiment 2, utilize method a to carry out carbon layer coating
[0131] The difference with Example 1 is:
[0132] Weigh 0.9g LiFePO 4 (5.70mmol), and added 0.065g (0.591mmol) resorcinol solid powder in the reaction system.
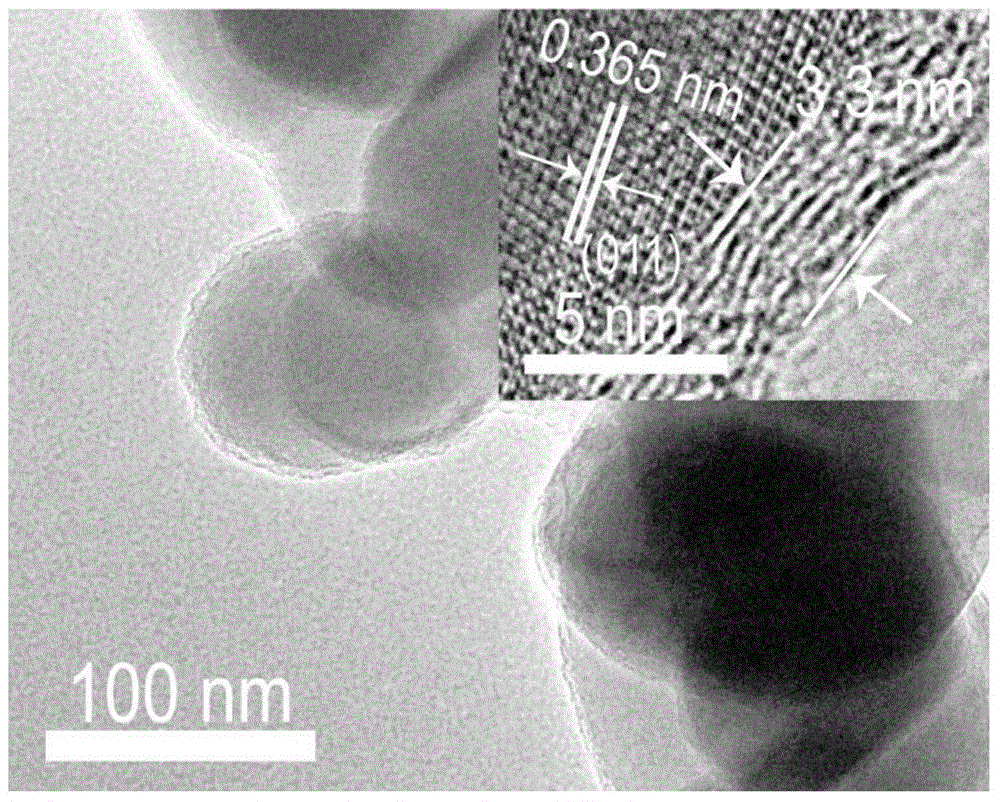
[0133] figure 2 It is the LiFePO prepared in this example 4 The transmission electron microscope (TEM) picture of C sample, as can be seen from the figure, the thickness of the coated carbon layer is 3.3nm, and this sample is denoted as LFPC (3.3).
Embodiment 3
[0134] Embodiment 3, utilize method a to carry out carbon layer coating
[0135] The difference with Example 1 is:
[0136] Weigh 0.4g LiFePO 4 (2.54mmol), and added 0.07g (0.636mmol) resorcinol solid powder in the reaction system.
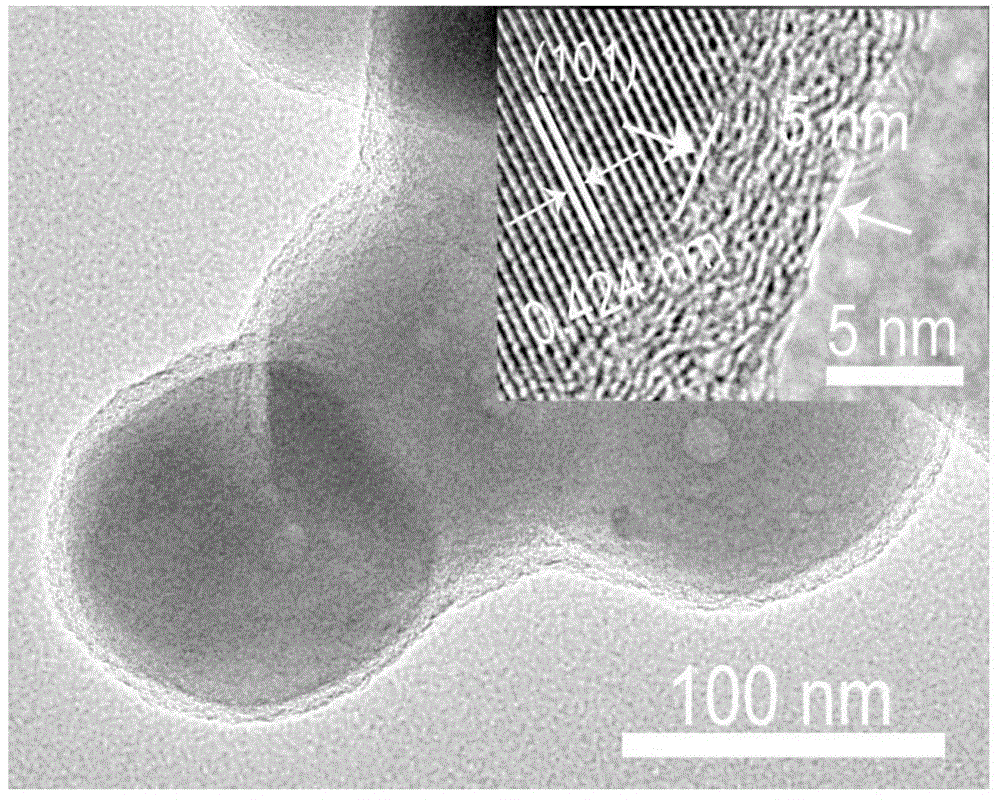
[0137] image 3 It is the LiFePO prepared in this example 4 The transmission electron microscope (TEM) figure of C sample, as can be seen from the figure, the thickness of the coated carbon layer is 5nm, and this sample is denoted as LFPC (5).
[0138] Figure 7 It is the XRD spectrogram of the LFPC sample prepared by the present embodiment, and intermediate product LFPRF and pure LiFePO 4 Compared with the standard spectrum, it can be seen that the synthesized material conforms to the standard card (JCPDS No.81-1173), orthorhombic crystal system, and Pnma space group. Carbon coated LiFePO 4 and LiFePO coated with phenolic resin polymer 4 and uncoated LiFePO 4 There is no difference in the spectrograms, indicating that the coating method ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Granularity | aaaaa | aaaaa |
| Granularity | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap