Method of treating alvelor bone loss through the use of anti-sclerostin antibodies
An anti-sclerostin and sclerostin technology, applied in the direction of antibody medical components, chemical instruments and methods, anti-growth factor immunoglobulin, etc., can solve problems such as alveolar bone defects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0139] This example describes different cell-based neutralization assays suitable for characterizing the neutralizing activity of anti-sclerostin antibodies.
[0140] MC3T3 Cell-Based Mineralization Assay - MC3T3-E1-BF cells were induced to differentiate using ascorbic acid and B-glycerophosphate, resulting in mineral deposition. An exemplary screening protocol in a 96-well format involved seeding cells on day 1, followed by 7 media changes over a 12-day period, with most mineral deposition occurring within the last 18 hours. The exact timing and extent of mineral deposition can vary depending in part on the particular serum lot used. Control experiments will allow these variables to be taken into account, as is generally known in the art of cell culture experimentation. For statistical analysis (using MS Excel and JMP), one-way ANOVA followed by Dunnett's comparison was used sequentially to determine differences between groups. Group means of data sets were considered signi...
Embodiment 2
[0155]The following examples illustrate the ability of a sclerostin inhibitor, anti-sclerostin monoclonal antibody (Scl-Ab), to enhance alveolar bone repair in a rat model of periodontal disease.
[0156] Rat periodontitis model: Experimental periodontal disease was induced in rats by ligation placement as previously described (Jin Q et al., 2007. J Periodontol 78:1300-1308; Graves, DT et al., 2008. J Clin Periodontol 35:89-105; the disclosure of which is incorporated herein by reference in its entirety). Briefly, male Sprague Dawley rats (approximately 300-350 g body weight, Harlan Laboratory, IN) were anesthetized with ketamine and thiazoline (83 / 17 ratio). A cotton suture (3.0) was then secured around the neck portion of the molar on one side of the mandible (just above the gum) to allow normal chewing. Assess the ligature three times a week, move the tip gently into the crevice to ensure subgingival placement, and replace if necessary.
[0157] Microcomputed tomography (...
PUM
| Property | Measurement | Unit |
|---|---|---|
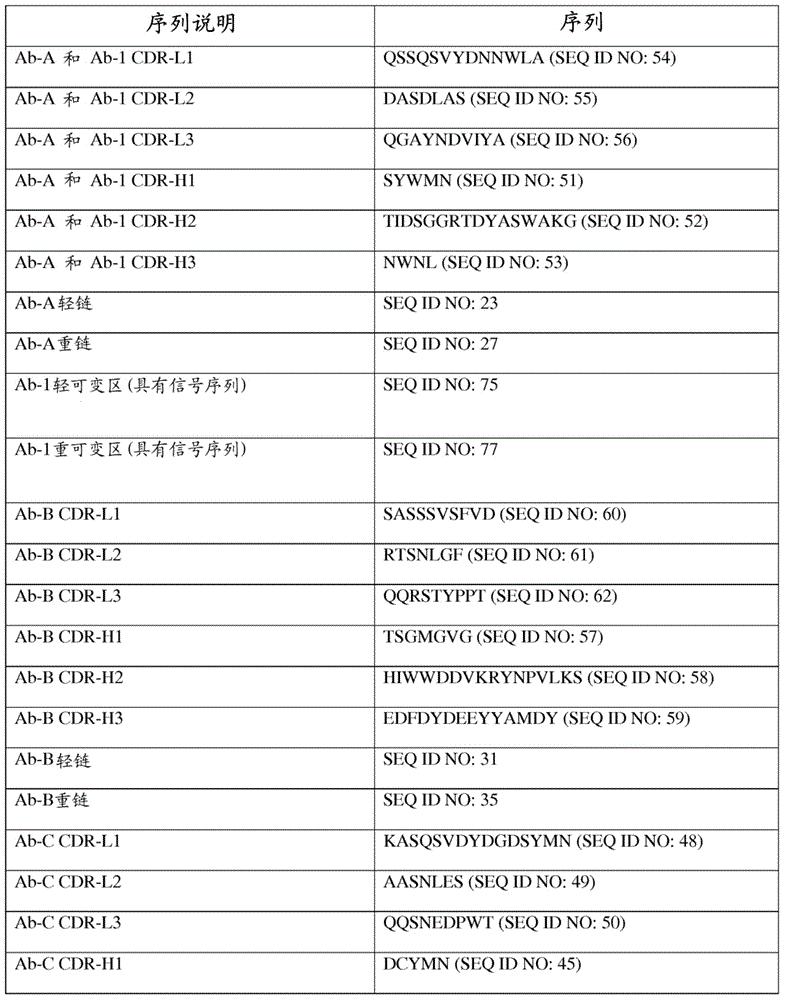
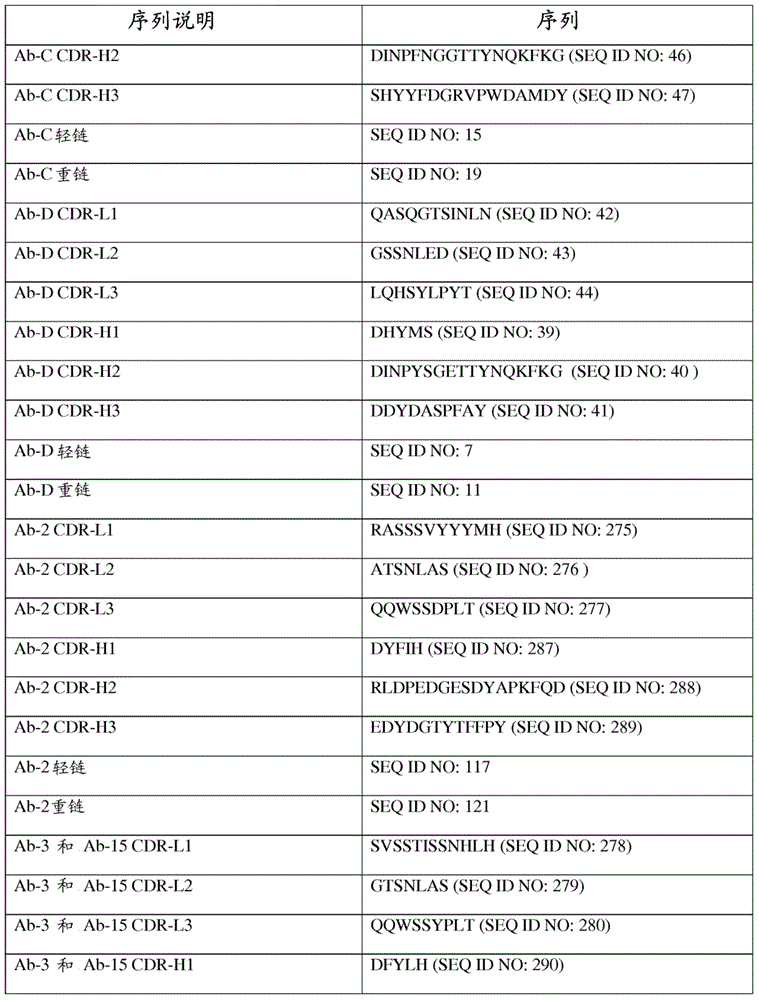
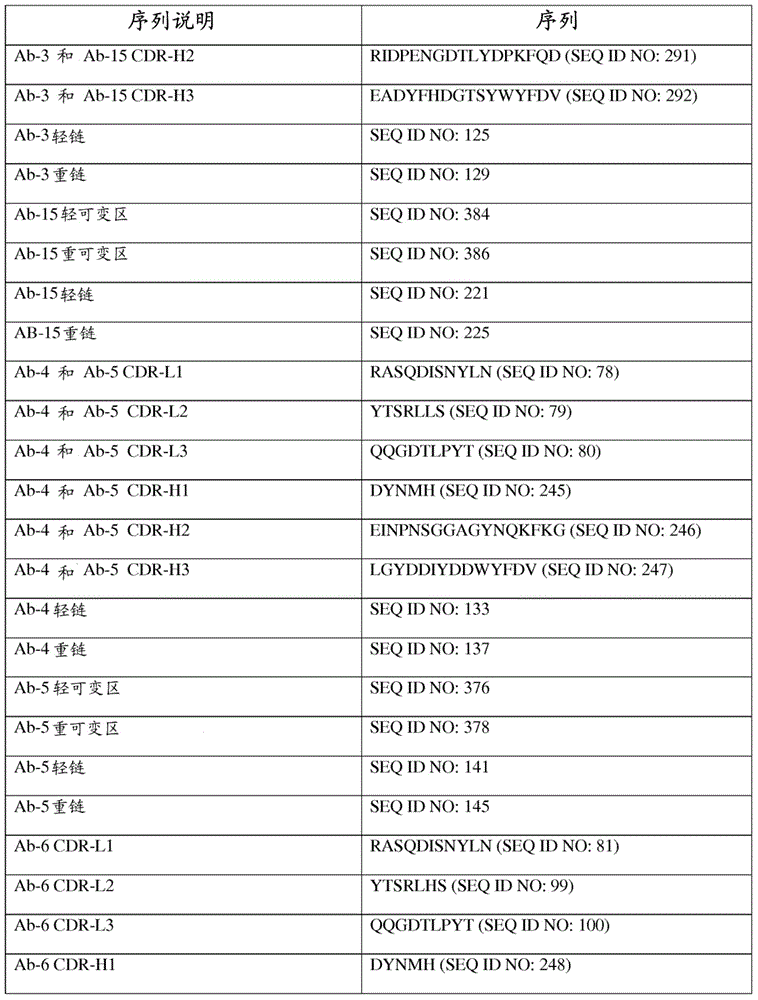
| affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com