Solid-state Lithium Ion Conductor And Electrochemical Device
A technology of electrochemical components and lithium ions, which is applied in the field of solid lithium ion conductors and electrochemical components, and can solve problems such as the improvement of ion conductivity that is not recorded
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1)
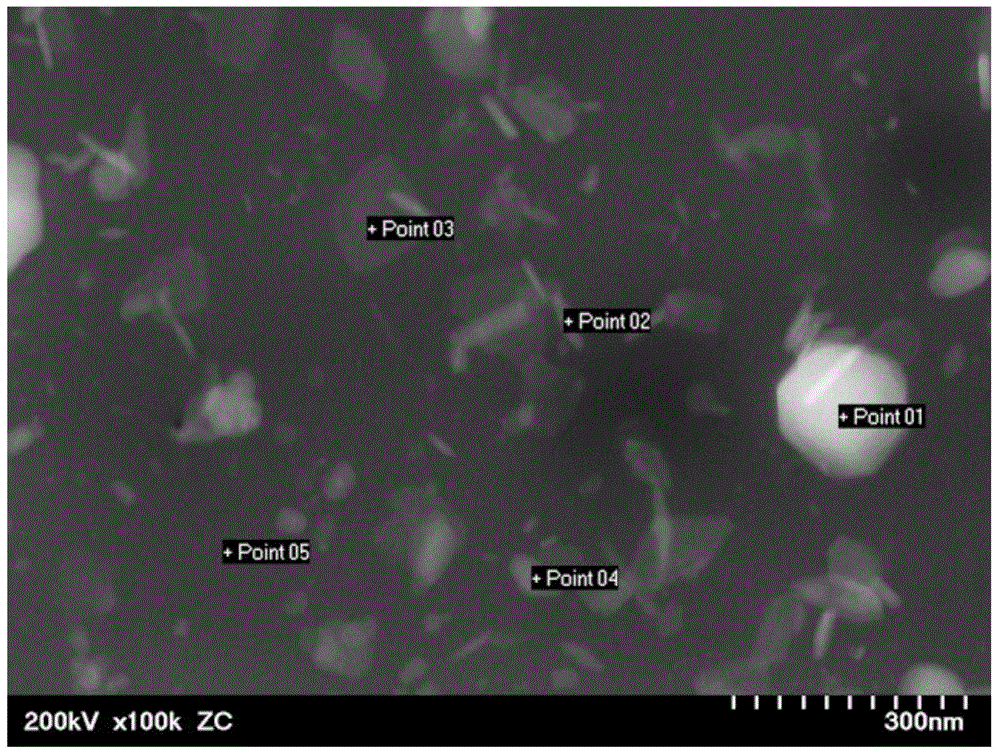
[0058] (sample making)
[0059] Weigh Li with a molar ratio of 85:15 2 S (manufactured by Japan High Purity Chemical Research Institute, model: LII06PB) and P 2 S 5 (manufactured by Aldrich, model: 232106), and 1 mol of ZnS (manufactured by Japan High Purity Chemical Research Institute, model: ZNI10PB) was weighed with respect to 99 mol of the mixture. Zn is divalent and contains 0.28 mol % of Zn based on all the materials weighed, and the molar ratio of Li to P is 5.7.
[0060] All the weighed materials were put into a planetary ball mill (manufactured by Fritsch), and pulverized and mixed at a rotation speed of 350 rpm for 6 hours.
[0061] After performing XRD measurement on the mixed powder, that is, the solid lithium ion conductor particles, there is no obvious diffraction peak, that is, there is no crystal phase, that is, an amorphous state. The solid lithium ion conductor particles were put into a tablet molding machine and compressed by the tablet molding machine t...
Embodiment 2)
[0065] The mixed powder obtained by pulverizing and mixing in the same manner as in Example 1 was heat-treated at 240° C. for 2 hours. After the XRD measurement of the mixed powder after the heat treatment, a plurality of obvious diffraction peaks appeared, thus confirming the formation of a crystal phase. After measuring the ionic conductivity in the same manner as in Example 1, the measured value obtained was 4.8×10 -4 S / cm. In addition, after measuring the electron conductivity by the direct current method, the measured value obtained was 3.4×10 -8 S / cm, thus electron conductivity is a negligible level.
Embodiment 3)
[0067] Weigh Li in a molar ratio of 85:15 2 S and P 2 S 5 and mix it. Weigh 0.5 moles of La with respect to 99.5 moles of the mixture 2 S 3 (manufactured by Japan High Purity Chemical Research Institute, model: LAI07PB). La is trivalent, contains 0.28 mol % of La of all the materials weighed, and the molar ratio of Li to P is 5.7. The weighed material was pulverized and mixed in the same manner as in Example 1.
[0068] After the XRD measurement of the mixed powder, that is, the solid lithium ion conductor particles, no obvious diffraction peaks appear, and it is a state without a crystal phase, that is, an amorphous state.
[0069] After measuring the ionic conductivity in the same manner as in Example 1, the measured value obtained was 3.5×10 -4 S / cm. In addition, after measuring the electron conductivity by the direct current method, the measured value obtained was 2.6×10 -8 S / cm, thus electron conductivity is a negligible level.
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical conductivity | aaaaa | aaaaa |
| electrical conductivity | aaaaa | aaaaa |
| electrical conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com