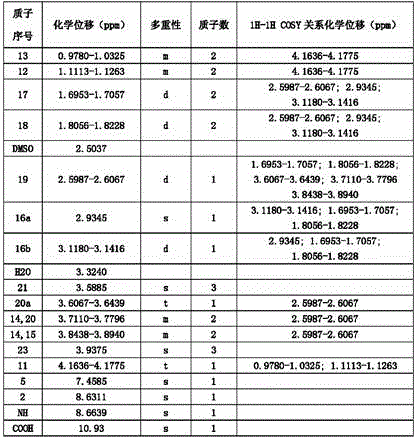
A method for preparing moxifloxacin impurity b and impurity d
A technology for moxifloxacin and impurities, which is applied in the field of medicinal chemistry, can solve problems such as an efficient synthesis method for impurity B of moxifloxacin, which is not seen, and achieve the effects of short synthesis route and reduction of by-products.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
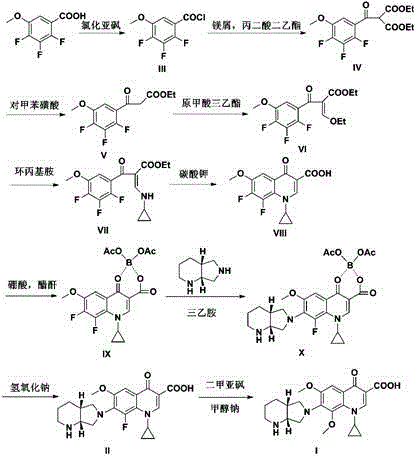
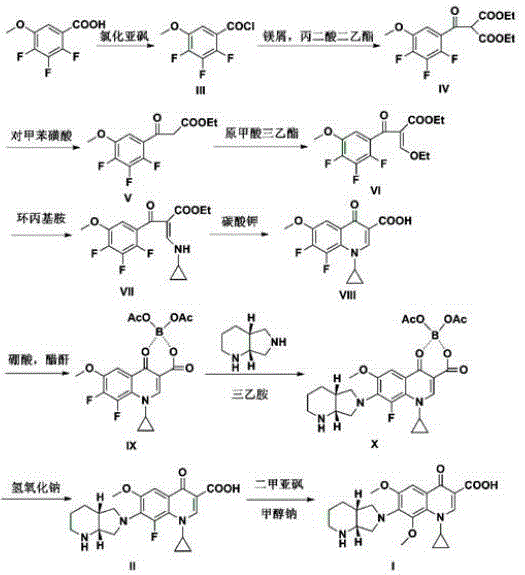
[0039] The preparation of embodiment 1 formula III compound
[0040] Stir and heat the mixture of 2,3,4-trifluoro-5-methoxybenzoic acid (82.5g, 0.4mol) and 350mL of thionyl chloride, and add about 1mL of catalytic amount of dimethylformamide DMF dropwise. Reflux at 90-95°C for 2.5 hours, concentrate the reaction solution to dryness, and obtain 86.6 g of a yellow oil (Formula III), which is dissolved by adding about 80 mL of anhydrous toluene to obtain an acid chloride solution, which will be used in the next reaction.
Embodiment 2
[0041] The preparation of embodiment 2 formula IV compound
[0042] In the mixture of magnesium chips (14.4g, 0.6mol) and absolute ethanol 56mL, add 7.2g of carbon tetrachloride and stir evenly; when there are bubbles in the mixture, slowly add diethyl malonate ( 76.8g, 0.48mol) mixed with toluene 180mL and absolute ethanol 50mL, control the temperature at 55-60°C for 2-3 hours, cool down to 0-5°C, slowly add the acid chloride (Formula III) solution in the step dropwise, After dropping, raise the temperature to 30-35°C to react for 2 hours. After the reaction is completed, slowly add 100 mL of 1 mol / L dilute hydrochloric acid dropwise, stir for 0.5 hours, let stand to separate layers, take the toluene layer, remove the toluene after drying, and obtain 123 g of oil (formula IV compound). MS (ESI+): 349.3 [M+H] +
Embodiment 3
[0043] The preparation of embodiment 3 formula V compound
[0044] Dissolve 123g of the compound of formula IV in 100mL of DMF, add 600mL of 1.5% p-toluenesulfonic acid aqueous solution, stir at 100-110°C for 10h, after the reaction is completed, cool down to room temperature, add 300mL of ethyl acetate and stir for 0.5h, let stand to separate layers, take acetic acid The ethyl acetate layer was dried and the ethyl acetate was removed to obtain 107 g of an oil (compound of formula V).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com