α, the method for preparing cumene by direct hydrogenolysis of α-dimethylbenzyl alcohol
A technology of dimethylbenzyl alcohol and cumene, applied in the field of alpha, can solve the problems of unstable catalyst, large amount of precious metal, environmental pollution and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
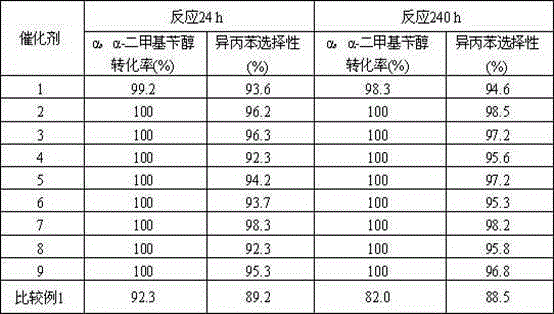
[0019] 100.0g activated carbon (specific surface area 1000m 2 / g) soaked in 5mol / LHNO 3 24 hours, then washed to pH 7.0 and then dried, 250 o Carrier I is obtained by roasting in C air; 1.0gPdCl 2 The solid was dissolved in a solution consisting of 10 g of concentrated hydrochloric acid, 19.0 g of nickel nitrate hexahydrate, and 70.0 g of purified water to obtain solution I. 50.0g solution I was impregnated on 50.0g carrier I, after drying at 250 o Catalyst 1 was obtained by calcining at C for 4 h. Its composition is: 0.57 parts of Pd-4.62 parts of NiO-94.8 parts of C.
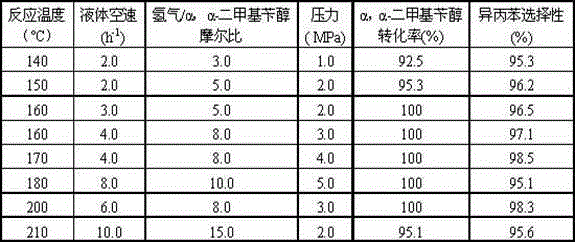
[0020] Catalyst 1 is loaded into the fixed bed reactor, and the filling capacity is 40.0ml, after H 2 250 o C for 4 hours. Containing α, the α-dimethylbenzyl alcohol hydrocarbon material contains 25% α, α-dimethylbenzyl alcohol and 75% cumene by weight percentage. The reaction process conditions are: 150°C of reaction temperature, 2.0MPa of reaction pressure, H 2 / α, the molar ratio of α-dimethylbenzy...
Embodiment 2
[0022] 100.0g activated carbon (specific surface area 1000m 2 / g) soaked in 5mol / LHNO 3 24 hours, then washed to pH 7.0 and then dried, 200 o Carrier I is obtained by roasting in C air; 2.0gPdCl 2 The solid was dissolved in a solution consisting of 10 g of concentrated hydrochloric acid, 19.0 g of cobalt nitrate hexahydrate, and 69.0 g of purified water to obtain solution I. 50.0g solution I was impregnated on 50.0g carrier I, after drying at 250 o The catalyst 2 was obtained by calcining at C for 4 h. Its composition is: 1.12 parts of Pd-4.60 parts of CoO-94.28 parts of C.
[0023] Catalyst 2 is packed into fixed-bed reactor, and the filling capacity is 40.0ml, and through H 2 300 oC for 4 hours. Containing α, the α-dimethylbenzyl alcohol hydrocarbon material contains 25% α, α-dimethylbenzyl alcohol and 75% cumene by weight percentage. The reaction process conditions are: 150°C of reaction temperature, 2.0MPa of reaction pressure, H 2 / α, the molar ratio of α-dimethy...
Embodiment 3
[0025] 100.0g activated carbon (specific surface area 1000m 2 / g) soaked in 5mol / LHNO 3 24 hours, then washed to pH 7.0 and then dried, 200 o Carrier I is obtained by roasting in C air; 1.0gPdCl 2 The solid was dissolved in 10g of concentrated hydrochloric acid, 15.0g of SnCl 4 ·5H 2 O, obtain solution I in the solution that 74.0g pure water forms. 50.0g solution I was impregnated on 50.0g carrier I, after drying at 250 o Catalyst 3 was obtained by calcining at C for 4 h. Its composition is: 0.56 parts of Pd-6.02 parts of SnO 2 -93.42 parts of C.
[0026] Catalyst 3 is packed into fixed-bed reactor, and the filling capacity is 40.0ml, through H 2 300 o C for 4 hours. Containing α, the α-dimethylbenzyl alcohol hydrocarbon material contains 25% α, α-dimethylbenzyl alcohol and 75% cumene by weight percentage. The reaction process conditions are: 170°C of reaction temperature, 2.0MPa of reaction pressure, H 2 / α, the molar ratio of α-dimethylbenzyl alcohol is 8.0, and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com