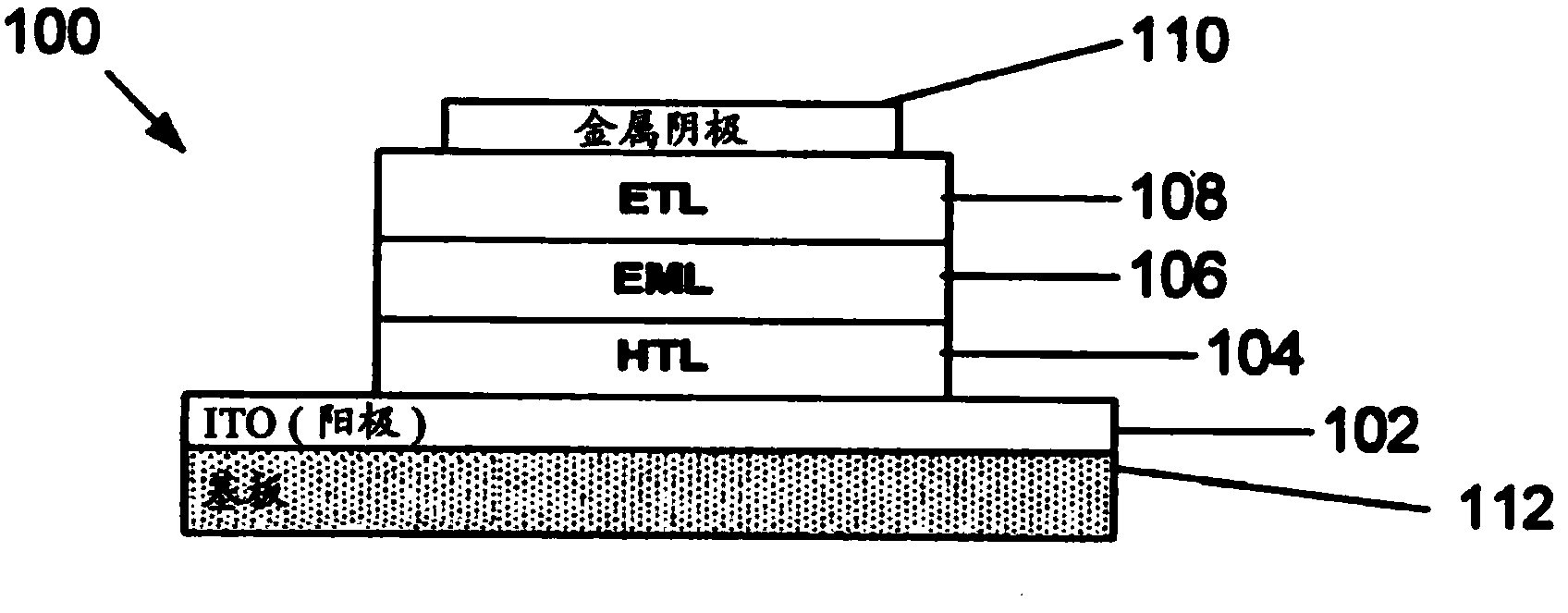
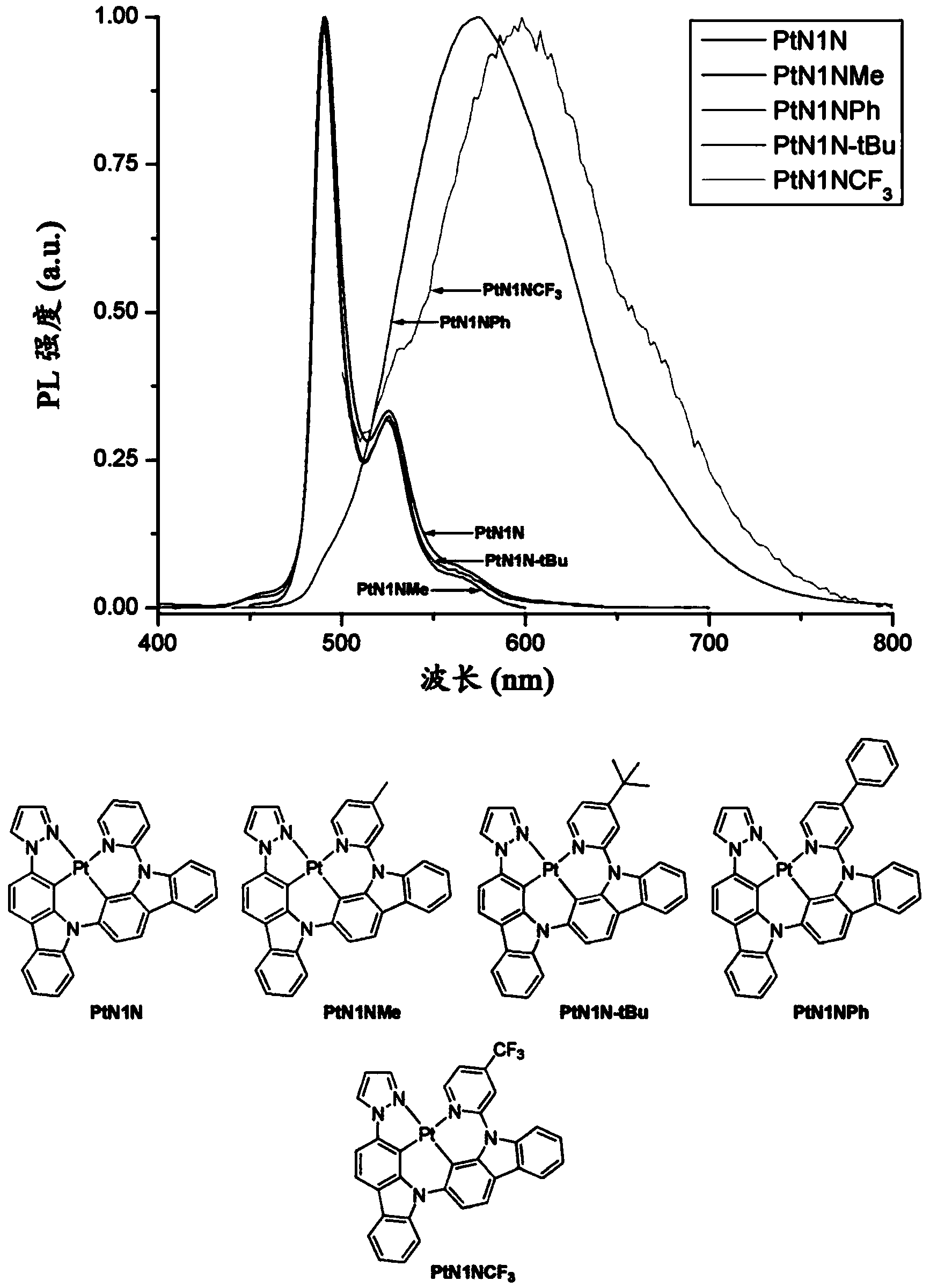
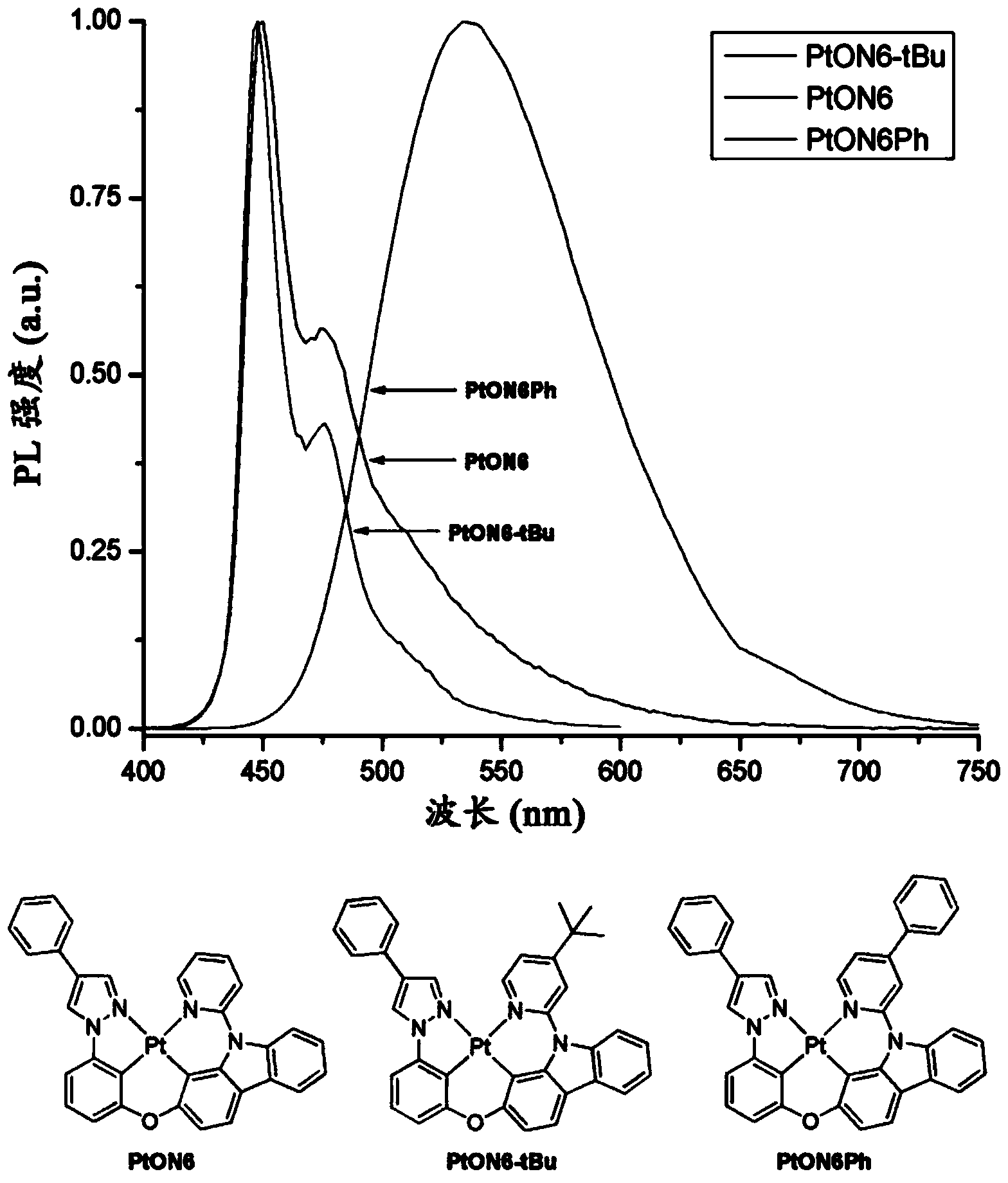
Phosphorescent tetradentate metal complexes having modified emission spectra
A compound and composition technology, applied in the field of phosphorescent tetradentate metal complexes with improved emission spectrum, can solve the problems of low stability, low-efficiency luminescence or absorption, poor processability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0159] The platinum complex PtN1N can be prepared according to the following scheme:
[0160]
[0161] Synthesis of 2-bromo-9H-carbazole 1:
[0162]
[0163] 4'-Bromo-2-nitrobiphenyl (22.40g, 80.55mmol) and P(OEt) 3 (150 mL) was added to a three-neck round bottom flask equipped with a magnetic stir bar and condenser under nitrogen. The mixture was heated to 150°C-160°C in an oil bath for 30 hours, cooled to ambient temperature, and excess P(OEt) was removed by distillation under high vacuum 3 . The residue was recrystallized in toluene to give the desired product 2-bromo-9H-carbazole (8.30 g) as white crystals. The filtrate was concentrated and the residue was purified by column chromatography on silica gel using hexane and ethyl acetate (10:1-5:1) as eluents to obtain the desired product 2-bromo-9H-carbazole 1 (2.00 g, 52% yield). 1 H NMR (DMSO-d 6 ,400MHz): δ7.17(t,J=7.6Hz,1H),7.28(dd,J=8.0,1.6Hz,1H),7.41(t,J=7.6Hz,1H),7.49(d,J= 8.4Hz, 1H), 7.65(d, J=1.6Hz, 1H),...
Embodiment 2
[0183] The platinum complex PtN1NMe can be prepared according to the following scheme:
[0184]
[0185] Synthesis of 9-(2'-nitrobiphenyl-4-yl)-2-(1H-pyrazol-1-yl)-9H-carbazole 6:
[0186]
[0187] 2-(1H-pyrazol-1-yl)-9H-carbazole 5 (1.35g, 5.78mmol, 1.0 equivalent), 4'-iodo-2-nitrobiphenyl 3 (2.26g, 6.94mmol, 1.2 Equivalent), K 3 PO 4 (2.58g, 12.14mmol, 2.1eq) and CuI (55mg, 0.29mmol, 0.05eq) were added to a dry pressure tube equipped with a magnetic stir bar. The tube was evacuated and then backfilled with nitrogen. The evacuation and backfilling process was repeated twice, then 1,2-cyclohexanediamine (0.33 g, 290 uL, 2.89 mmol, 0.5 equiv) and dioxane (20 mL) were added. The mixture was bubbled with nitrogen for 30 minutes and the tube was sealed. The mixture was stirred for 3 days in an oil bath at a temperature of 110-120°C and then cooled to ambient temperature. The solvent was removed under reduced pressure and the residue was purified by column chromatograph...
Embodiment 3
[0198] The platinum complex PtN1N-tBu can be prepared according to the following scheme:
[0199]
[0200] Synthesis of 9-(4-tert-butylpyridin-2-yl)-2'-(1H-pyrazol-1-yl)-9H-2,9'-dicarbazole ligand N1N-tBu:
[0201]
[0202] 2'-(1H-pyrazol-1-yl)-9H-2,9'-dicarbazole 7 (398mg, 1.0mmol, 1.0eq), Pd 2 (dba) 3 (46mg, 0.05mmol, 0.05 equivalent), JohnPhos (30mg, 0.1mmol, 0.1 equivalent) and t BuONa (154 mg, 1.6 mmol, 1.6 equiv) was added to a dry pressure Schlenk tube equipped with a magnetic stir bar. The tube was then evacuated and backfilled with nitrogen. The process of evacuation and backfilling was repeated twice, then anhydrous toluene (4 mL), dioxane (4 mL) and 2-bromo-4-tert-butylpyridine (418 mg, 2.0 mmol, 2.0 equiv) were added under nitrogen . The tube was sealed and the mixture was stirred for 2 days in an oil bath at a temperature of 95-105°C. The mixture was then cooled to ambient temperature. The solvent was removed under reduced pressure and the residue was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com