Preparation and application of fusion protein and vaccine composition thereof
A vaccine composition and fusion protein technology, applied in the field of veterinary biological products, can solve problems such as sudden onset, high morbidity and mortality, and economic loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] Example 1 Preparation, identification and content determination of fusion protein 1
[0074] According to the COE neutralization table of the S protein fragment in the porcine epidemic diarrhea virus (accession number: JN599150.1, AB857233.1, AB857234.1) reported in NCBI (http: / / www.ncbi.nlm.nih.gov) The gene sequence of the position (see SEQ ID No. 1, SEQ ID No. 3 (artificial synthesis), SEQ ID No. 5, SEQ ID No. 7), the gene sequence of the neutralizing epitope of the combination of SS2-SS6-2C10 (SEQ ID No. 9), and the gene sequence of the Fc protein in porcine immunoglobulin IgG (accession number: NM_213828.1) (see SEQ ID No. 11) to prepare the fusion protein by genetic engineering.
[0075] 1.1 Construction and identification of antigenic protein of porcine epidemic diarrhea virus
[0076] When constructing the COE neutralizing epitope in the S protein fragment of porcine epidemic diarrhea virus, specific restriction sites such as EcoRI and HindIII were introduced at the 5...
Embodiment 2
[0091] Example 2 Preparation of porcine epidemic diarrhea virus vaccine composition
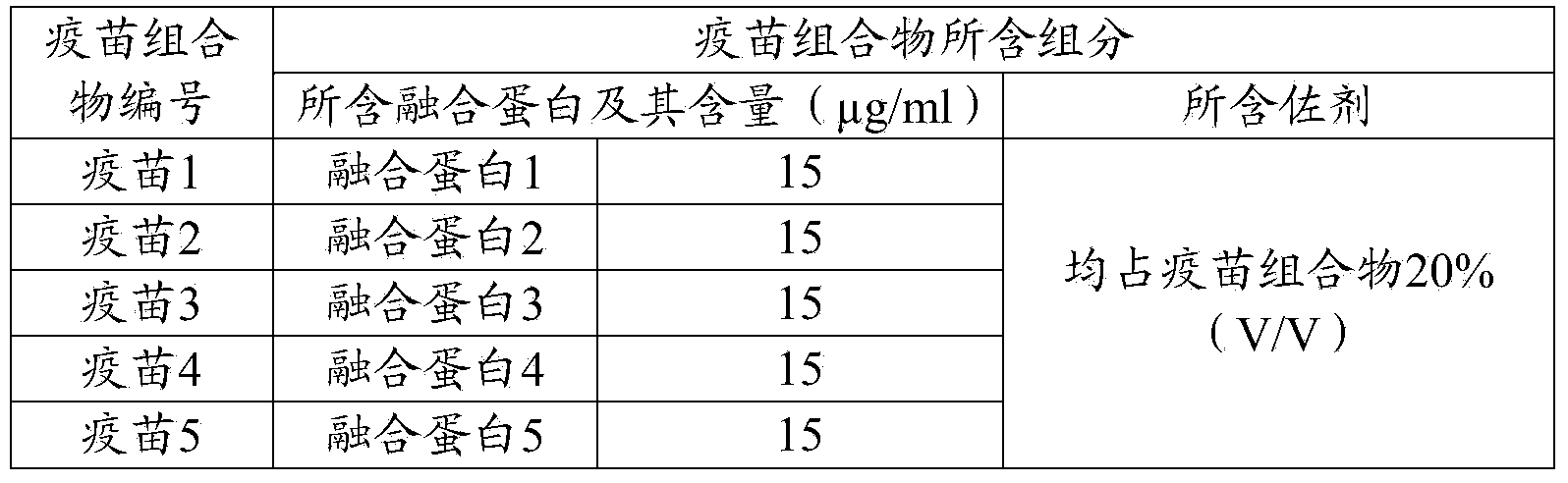
[0092] Use pH 7.4PBS to dilute the fusion protein prepared in Example 1, and add aluminum gel adjuvant to mix well so that the content of the recombinant fusion protein contained in the vaccine composition is shown in Table 1, while ensuring the aluminum gel adjuvant The volume ratio to the vaccine composition is 1:5. The prepared vaccine composition is used as an immunogen and stored at 4°C for later use.
[0093] Table 1 Components contained in porcine epidemic diarrhea vaccine composition
[0094]
Embodiment 3
[0095] Example 3 Evaluation of immune efficacy of porcine epidemic diarrhea vaccine composition
[0096] 3.1 Active immunity test
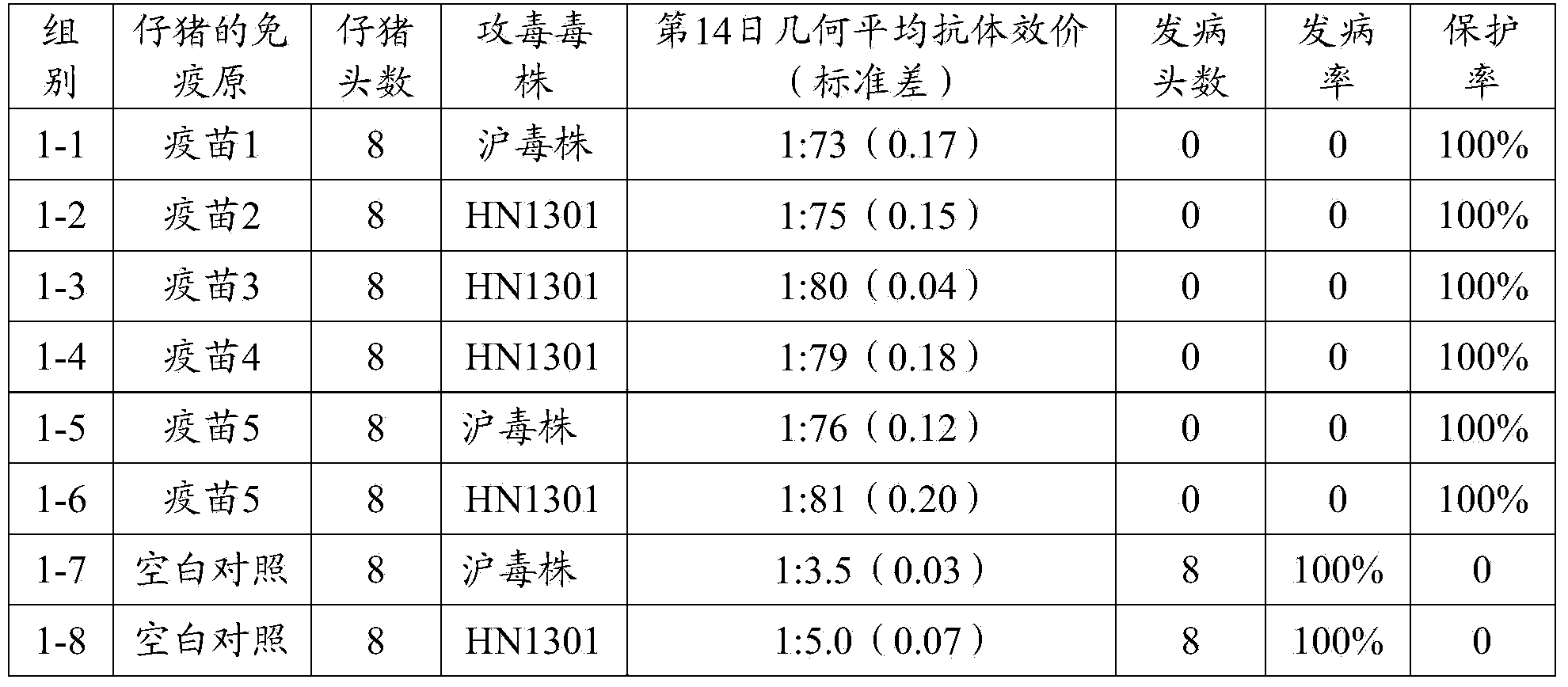
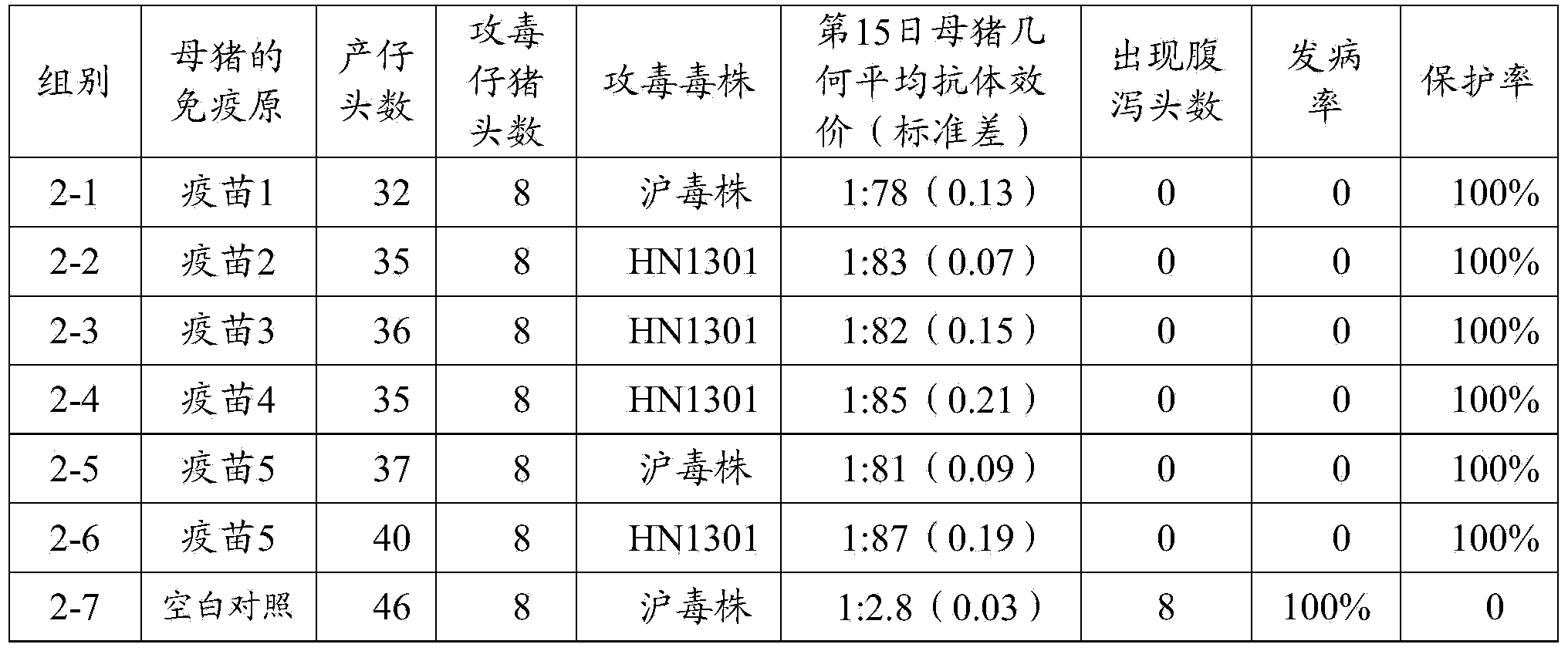
[0097] A total of 64 3-day-old PEDV antibody and antigen-negative piglets were selected and randomly divided into 8 groups (see Table 2 for details), 8 pigs per group. Groups 1-1, 1-2, 1-3, 1-4, 1-5, and 1-6 were injected intramuscularly with the vaccine prepared in Example 2 1-5, 2ml / head; No. 1-7, 1- The 8 groups were all blank control groups immunized with the same dose of pH7.4 PBS solution. On the 14th day after immunization, sera from the immunized group and the control group were collected, and the serum neutralizing antibody titer of each group was detected. The SPSS 15.0 software was used for statistical analysis of the average antibody titer of each piglet. At the same time, in the immune group, except for the 1-1, 1-5 and blank control groups, the pigs in groups 1-7 were given 1ml of Shanghai virus strain virus liquid (viral liquid TCID 5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com