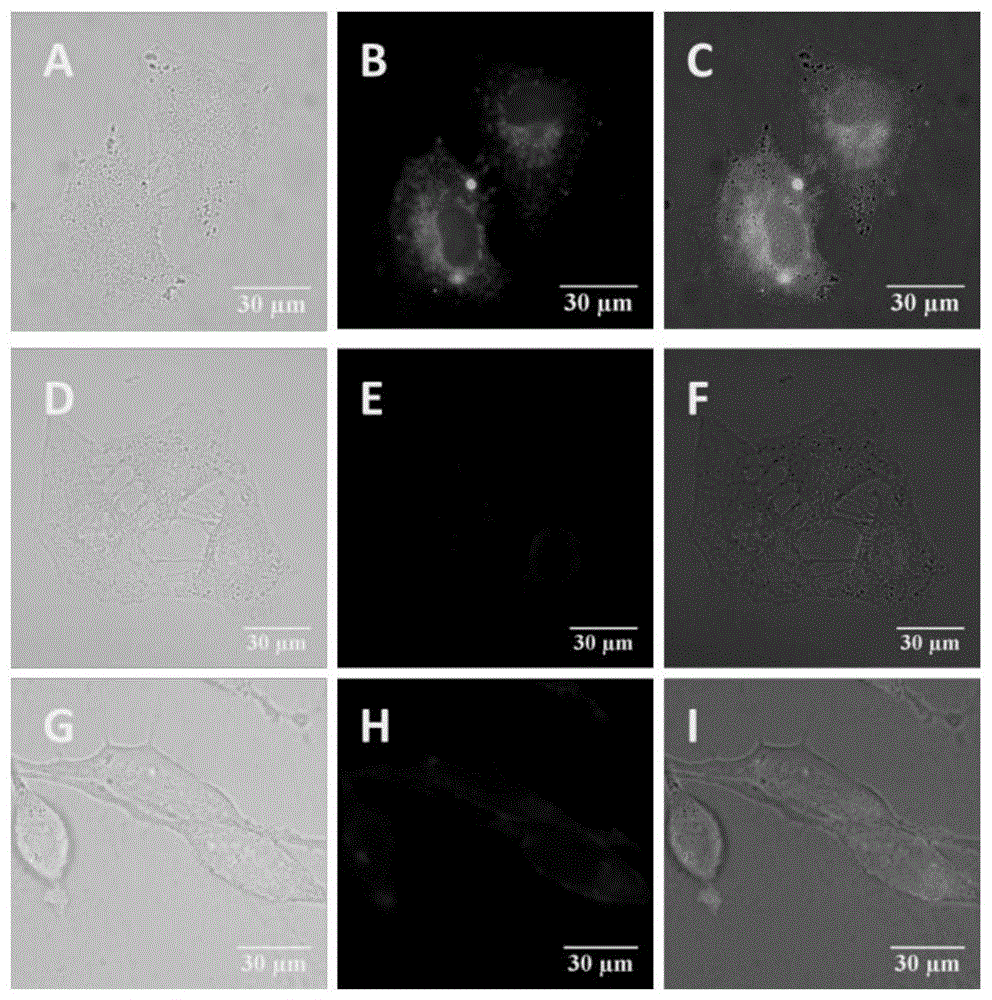
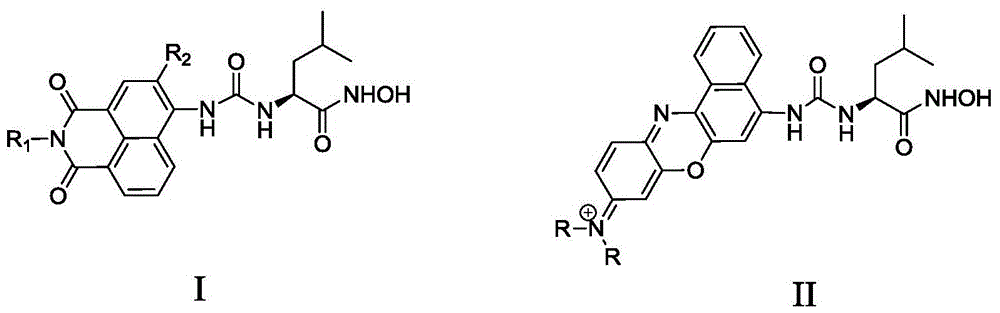
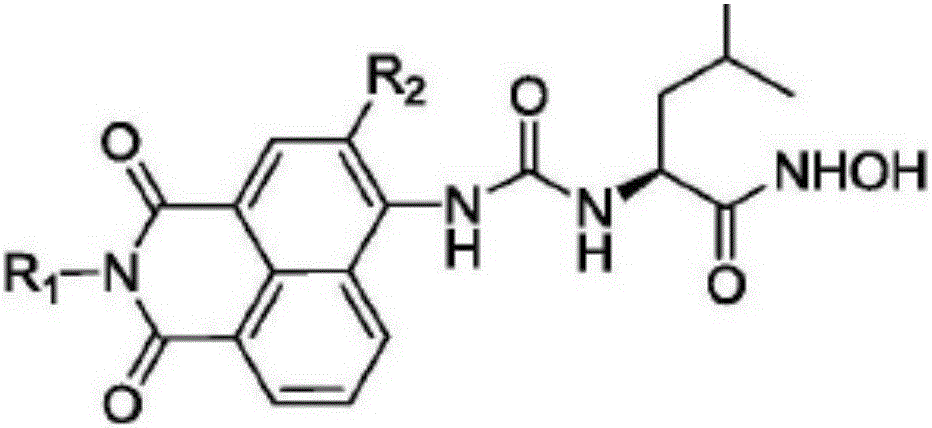
A kind of APN inhibitor with fluorescent property and its application
A technology of inhibitors and properties, applied in the field of APN inhibitors, can solve the problem of weak binding between inhibitors and APN active centers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Preparation of L1-11
[0071] synthetic route:
[0072]
[0073] Concrete synthetic steps are as follows:
[0074] Intermediate 1a: 6-Amino-1H-benzo[de]isoquinoline-1,3(2H)-dione
[0075] Suspend 4-amino-1,8-naphthalic anhydride (0.30 g, 1.4 mmol) in 28% NH 3 -H 2 In O (30 mL), reflux for 5 hours, concentrate to dryness, and obtain a brown powder after drying, 0.2 g, yield 67.0%. It was directly used in the next step without purification.
[0076] Intermediate 1b: 6-Amino-2-methyl-1H-benzo[de]isoquinoline-1,3(2H)-dione
[0077] Suspend 4-amino-1,8-naphthalene dicarboxylic anhydride (0.30g, 1.4mmol) in ethanol solution (30mL) with a volume concentration of 30% methylamine, reflux for 5 hours, concentrate to dryness, and obtain a brown color after drying Powder, 0.26g, yield 85.7%. It was directly used in the next step without purification.
[0078] Intermediate 1c: 6-Amino-2-ethyl-1H-benzo[de]isoquinoline-1,3(2H)-dione
[0079] Suspend 4-amino-1,8-naphthalic ...
Embodiment 2
[0142] (S)-2-(3-(5-Bromo-2-methyl-1,3-dioxo-2,3-dihydro-1H-benzo[de]isoquinolin-6-yl)urea base)-N-hydroxyl-4-methylpentanamide [L13]
[0143] synthetic route
[0144]
[0145] Intermediate 3b: (S)-Methyl 2-(3-(5-bromo-2-methyl-1,3-dioxo-2,3-dihydro-1H-benzo[de]isoquinoline -6-yl)ureido)-4-methylpentanamide
[0146] Intermediate 2b (50mg, 0.125mmol) was dissolved in 20mL of acetonitrile, N-bromosuccinimide NBS (26.7mg, 0.15mmol) was added, stirred at room temperature for 24 hours, the solution became turbid, no 2b remained by TLC, filtered The off-white powder was obtained, 55mg, yield 92.6%, melting point 213.8-214.5°C. 1 HNMRδ H (DMSO-d 6 ,300MHz)8.72(s,1H),8.55(s,1H),8.51(dd,J=7.2,Hz,0.9Hz,1H),8.25(dd,J=8.4,Hz,0.9Hz,1H),7.88 (dd,J=8.4,Hz,7.2Hz,1H),7.14(d,J=8.1Hz,1H),4.24-4.32(m,1H),3.68(s,3H),3.39(s,3H), 1.71-1.80 (m, 1H), 1.56-1.63 (m, 2H), 0.94 (t, J=6.9Hz, 6H).
[0147] Inhibitor L13: (S)-2-(3-(5-bromo-2-methyl-1,3-dioxo-2,3-dihydro-1H-benzo[de]isoquinoline-6 ...
Embodiment 3
[0150] (S)-2-(3-(5-Bromo-2-methyl-1,3-dioxo-2,3-dihydro-1H-benzo[de]isoquinolin-6-yl)urea base)-N-hydroxyl-4-methylpentanamide [L24]
[0151] synthetic route
[0152]
[0153] Intermediate 4b: (S)-Methyl 2-(3-(5-chloro-2-methyl-1,3-dioxo-2,3-dihydro-1H-benzo[de]isoquinoline -6-yl)ureido)-4-methylpentanamide
[0154] Dissolve 2b (160 mg, 0.4 mmol) in 5 mL of acetonitrile, add N-chlorosuccinimide NCS (63.8 mg, 0.48 mmol), add 1 drop of concentrated hydrochloric acid under stirring, turbidity occurs within 3 minutes, and 2b after 5 minutes The response is complete. The yellow-white powder was obtained by filtration, 80mg, yield 46.4%, melting point 227.2-228.0°C. 1 HNMRδ H (DMSO-d 6,400MHz)8.81(s,1H),8.49(d,J=6.8,Hz,1H),8.41(s,1H),8.25(d,J=8.4,Hz,1H),7.89(t,J=8.4 ,Hz,1H),7.13(d,J=8.4Hz,1H),4.25-4.31(m,1H),3.68(s,3H),3.39(s,3H),1.71-1.78(m,1H), 1.55-1.66 (m, 2H), 0.94 (t, J = 6.9Hz, 6H).
[0155] Inhibitor L24: (S)-2-(3-(5-Chloro-2-methyl-1,3-dioxo-2,3-dihydro-1H-benzo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com