A kind of preparation method of low melting point regenerated copolyester
A technology of copolyester and low melting point, applied in the field of preparation of low melting point regenerated copolyester, can solve problems such as low catalytic efficiency of catalyst, and achieve the effect of good economic value and environmental protection value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
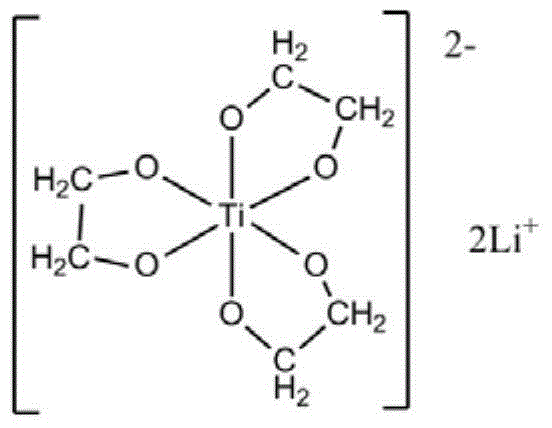
[0050] At first carry out the preparation of ethylene glycol lithium titanate, it comprises the following steps:
[0051] a) Uniformly mix tetraethyl titanate with ethylene glycol at 50°C under nitrogen protection, the molar ratio of tetraethyl titanate to ethylene glycol is 1:100;
[0052] b) Add lithium hydroxide, the molar ratio of lithium hydroxide to tetraethyl titanate is 2.05:1, keep stirring until the reaction system is a uniform and transparent liquid, then raise the reaction temperature to 180°C;
[0053] c) During the reaction process, reflux ethylene glycol and exclude the low-boiling ethanol and water generated by the reaction. The reaction time is 3 hours. Completely evaporate the ethylene glycol in the system, and completely evaporate the dihydric alcohol in the system to obtain a white solid, which is recrystallized three times with ethanol-chloroform to obtain a colorless crystal, which is to obtain ethylene glycol lithium titanate;
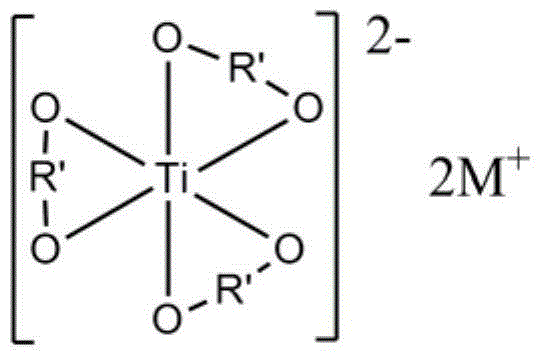
[0054] The structural fo...
Embodiment 2
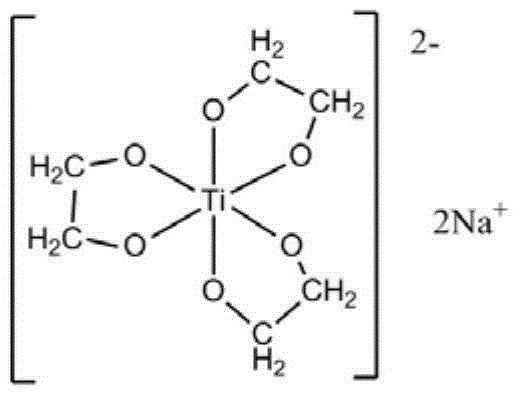
[0066] At first carry out the preparation of ethylene glycol sodium titanate, it may further comprise the steps:
[0067] a) Uniformly mix tetra-n-butyl titanate with ethylene glycol at 150°C under nitrogen protection, the molar ratio of tetra-n-butyl titanate to ethylene glycol is 1:50;
[0068] b) Add sodium hydroxide, the molar ratio of sodium hydroxide to tetra-n-butyl titanate is 2.03:1, continue to stir until the reaction system is a uniform and transparent liquid, then raise the reaction temperature to 210°C;
[0069] c) During the reaction process, reflux ethylene glycol and exclude the low-boiling n-butanol and water generated by the reaction. The reaction time is 2 hours. Completely evaporate the ethylene glycol in the system and completely evaporate the dihydric alcohol in the system to obtain a white solid, which is recrystallized three times with ethanol-chloroform to obtain a colorless crystal, which is to obtain ethylene glycol sodium titanate;
[0070] The str...
Embodiment 3
[0082] At first carry out the preparation of propylene glycol potassium titanate, it may further comprise the steps:
[0083] a) Uniformly mix tetraisopropyl titanate with 1,2-propanediol at 100°C under nitrogen protection conditions, the molar ratio of tetraisopropyl titanate to 1,2-propanediol is 1:80;
[0084] b) Add potassium hydroxide, the molar ratio of potassium hydroxide to tetraisopropyl titanate is 2.02:1, keep stirring until the reaction system is a uniform and transparent liquid, then raise the reaction temperature to 200°C;
[0085] c) During the reaction, reflux 1,2-propanediol and exclude the low-boiling isopropanol and water generated by the reaction. The reaction time is 4 hours. After the reaction is completed, the temperature is 200 ° C, and the reaction system pressure is 0.7 atm, 1 min Completely evaporate the ethylene glycol in the system, and completely evaporate the glycol in the system to obtain a light yellow solid, which is recrystallized three times...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com