Orally disintegrating tablet containing ezetimibe and preparation method of orally disintegrating tablet
A technology of oral disintegrating tablet and ezetimibe, which is applied in the field of ezetimibe oral disintegrating tablet and preparation thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Example 1 Preparation of ezetimibe orally disintegrating tablets
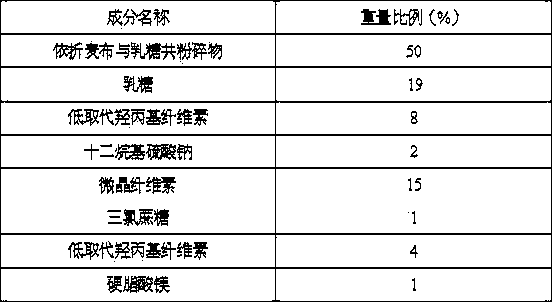
[0020] Each tablet contains 10mg of ezetimibe, and its prescription is:
[0021]
[0022] Preparation Process:
[0023] (1) Pass filler, disintegrant, flavoring agent, binder and lubricant through a 100-mesh sieve; ezetimibe through a 60-mesh sieve;
[0024] (2) Co-crushing ezetimibe and filler;
[0025] (3) Mix the pulverized product of ezetimibe and filler with disintegrant and flavoring agent, add binder to granulate, dry at 50°C, add lubricant, and press into tablets to obtain ezetimibe Orally disintegrating tablet.
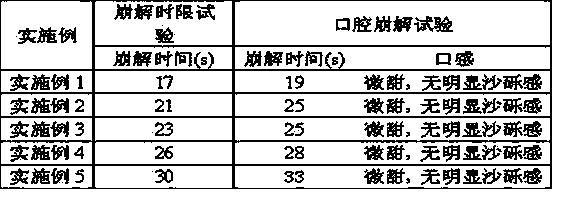
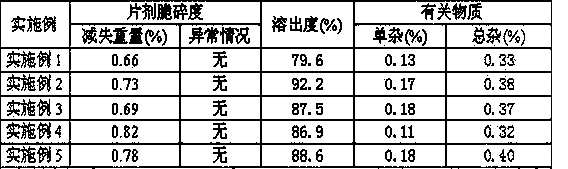
[0026] Each ezetimibe orally disintegrating tablet contains 10 mg of ezetimibe, weighs 100 mg, and has a hardness of 20N to 30N.
Embodiment
[0027] Example 2 Preparation of ezetimibe orally disintegrating tablets
[0028]
[0029] Preparation Process:
[0030] (1) Pass filler, disintegrant, flavoring agent, binder and lubricant through a 100-mesh sieve; ezetimibe through a 60-mesh sieve;
[0031] (2) Co-crushing ezetimibe and filler;
[0032] (3) Mix the pulverized product of ezetimibe and filler with disintegrant and flavoring agent, add binder to granulate, dry at 50°C, add lubricant, and press into tablets to obtain ezetimibe Orally disintegrating tablet.
[0033] Each ezetimibe orally disintegrating tablet contains 10 mg of ezetimibe, weighs 100 mg, and has a hardness of 20N to 30N.
[0034] Example 3 Preparation of ezetimibe orally disintegrating tablets
[0035]
[0036] Preparation Process:
[0037] (1) Pass filler, disintegrant, flavoring agent, binder and lubricant through a 100-mesh sieve; ezetimibe through a 60-mesh sieve;
[0038] (2) Co-crushing ezetimibe and filler;
[0039] (3) Mix t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap