Method for preparing 1,2-propanediol using biomass derivative lactic acid
A technology for derivatives and propylene glycol, applied in the field of preparing 1,2-propylene glycol, can solve the problems of low yield of 1,2-propylene glycol, harsh reaction conditions, depletion of reserves, etc., to reduce reaction energy consumption, reduce reaction by-products, The effect of less environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
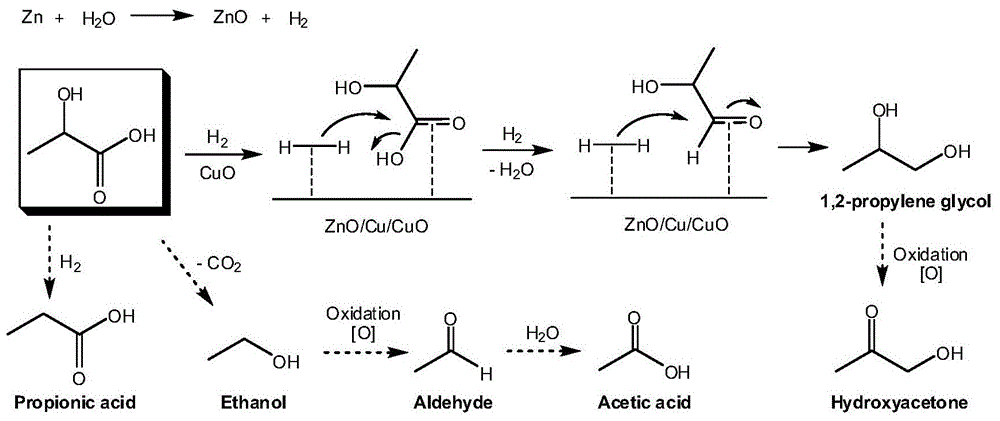
[0040] This example relates to a method for preparing 1,2-propanediol by hydrothermal conversion of metal element (Zn powder) and metal oxide (CuO powder) to lactic acid. The reaction equation is as follows:
[0041]
[0042] The method comprises the steps of:
[0043] Lactic acid (75mg, reaction concentration is 7.14g / L), Zn powder (30mmol) and CuO powder (5mmol) are packed in the hydrothermal reactor of Teflon liner successively, add water and make reactor filling rate 35%, to reaction Fill the reactor with nitrogen to eliminate the interference of air and seal it. Put the reactor into an oven to make the reaction temperature 250°C, and the reaction time is 120 minutes. After the reaction, take out the mixture and filter to obtain 1,2-propanediol.
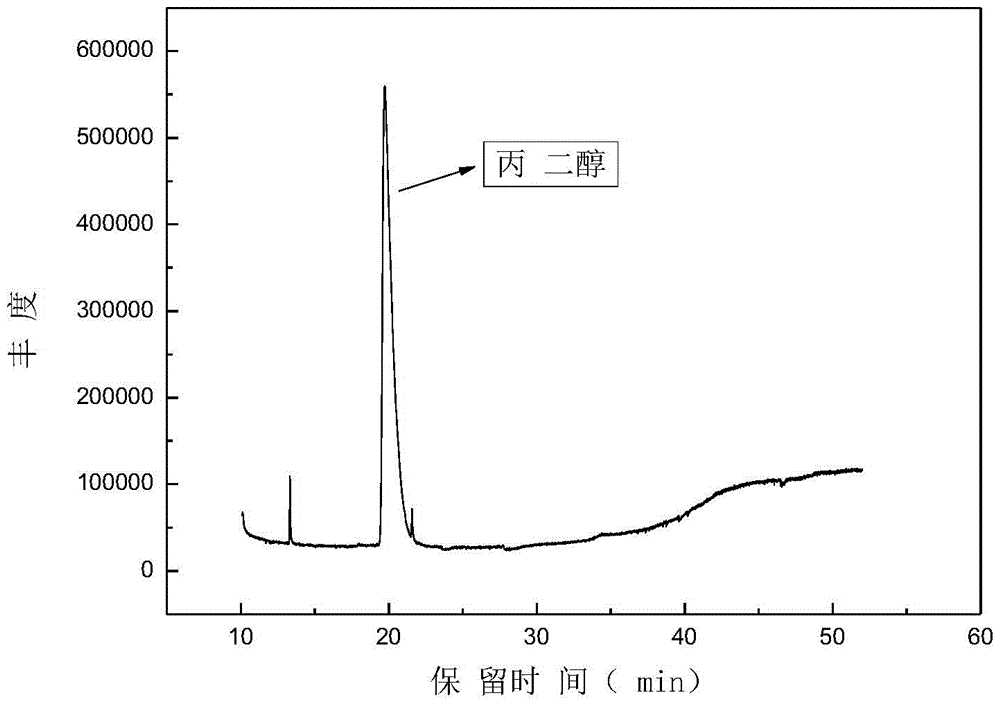
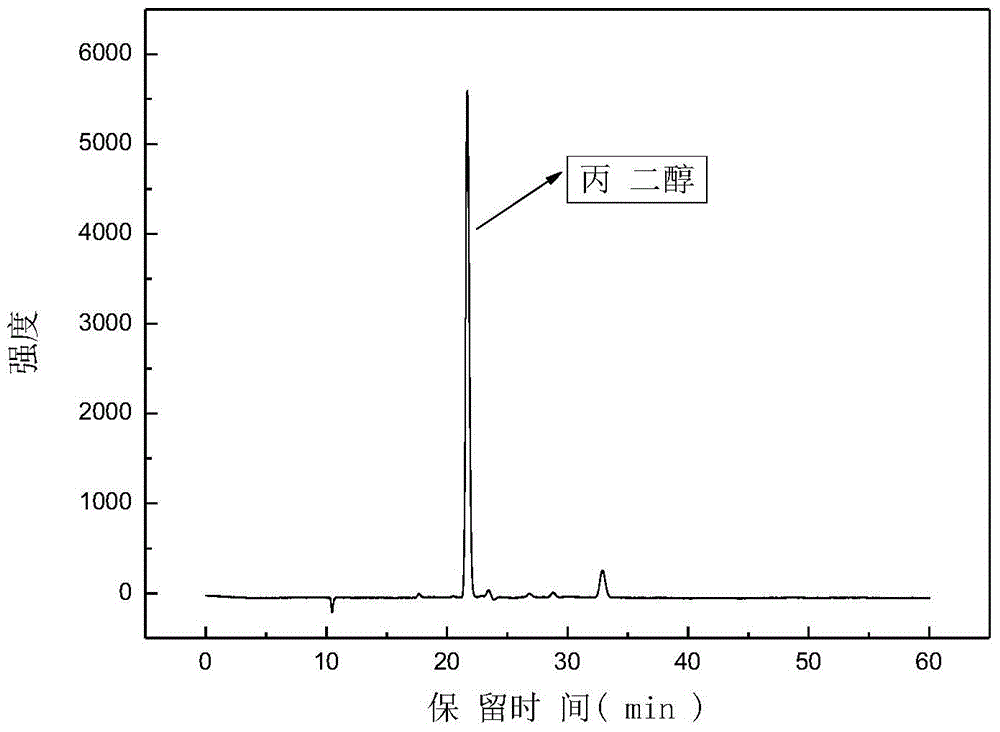
[0044] After the reaction, the product was characterized by GC / MS (see figure 1 ) and HPLC quantitative analysis (see figure 2 ), GC / MS qualitative analysis shows that 1,2-propanediol is the main product, and HPLC quantitat...
Embodiment 2
[0046] This embodiment relates to a method for preparing 1,2-propanediol by hydrothermal conversion of metal element (Zn powder) and metal oxide (CuO powder) to lactic acid. The method includes the following steps:
[0047] Lactic acid (75mg, reaction concentration is 10g / L), Zn powder (30mmol) and CuO powder (5mmol) are packed in the hydrothermal reactor of Teflon liner successively, add water to make the reactor filling rate 25%, add to reactor Fill the reactor with nitrogen to eliminate the interference of the air and seal it. Put the reactor into an oven to make the reaction temperature 250°C, and the reaction time is 120 minutes. After the reaction, take out the mixture and filter to obtain 1,2-propanediol.
[0048] GC / MS qualitative analysis and HPLC quantitative analysis were performed on the reacted product. GC / MS qualitative analysis showed that 1,2-propanediol was the main product, and HPLC quantitative analysis showed that the yield could reach up to 86%.
Embodiment 3
[0050] This embodiment relates to a method for preparing 1,2-propanediol by hydrothermal conversion of metal element (Zn powder) and metal oxide (CuO powder) to lactic acid. The method includes the following steps:
[0051] Lactic acid (75mg, reaction concentration is 25g / L), Zn powder (30mmol) and CuO powder (3mmol) are packed in the hydrothermal reactor of Teflon liner successively, add water to make the reactor filling rate 10%, add to reactor Fill the reactor with nitrogen to eliminate the interference of the air and seal it. Put the reactor into an oven to make the reaction temperature 250°C, and the reaction time is 120 minutes. After the reaction, take out the mixture and filter to obtain 1,2-propanediol.
[0052]GC / MS qualitative analysis and HPLC quantitative analysis were performed on the reacted product. GC / MS qualitative analysis showed that 1,2-propanediol was the main product, and HPLC quantitative analysis showed that the yield could reach up to 90%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com