A kind of stable limaprost pharmaceutical composition and preparation method thereof
A technology for limaprost and composition, which is applied in the field of limaprost composition and preparation thereof, can solve problems such as enhancement of hygroscopicity, and achieve the effects of reducing hygroscopicity, increasing selection of species and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] prescription:
[0028]
[0029] Preparation Process:
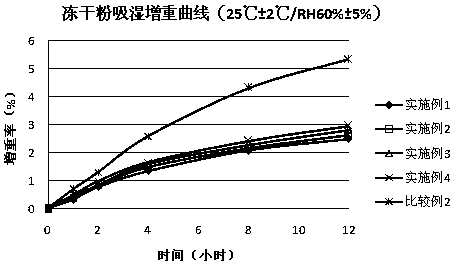
[0030] Weigh the inclusion compound of chitosan, trehalose, hydrogenated castor oil and limatoprost alfa cyclodextrin in the prescribed amount, disperse with appropriate amount of water, freeze-dry, the pre-freezing temperature is -35 ℃, and the primary drying temperature is - 10°C, the secondary drying temperature is 25°C; the freeze-dried lumps are pulverized and passed through an 80-mesh sieve to obtain a freeze-dried powder; Magnesium acid is mixed evenly and compressed into tablets.
Embodiment 2
[0032] prescription:
[0033]
[0034] Preparation Process:
[0035] Weigh the prescription amount of sucrose, dextrin, silicon dioxide and limatoprost alpha cyclodextrin inclusion compound, disperse with appropriate amount of tert-butanol-water system (volume ratio 1:30), freeze-dry, and pre-freeze the temperature -45°C, the primary drying temperature is -20°C, and the secondary drying temperature is 15°C; the freeze-dried block is pulverized and passed through an 80-mesh sieve to obtain a freeze-dried powder; then mixed with the prescribed amount of mannitol and microcrystalline Cellulose, croscarmellose sodium, pregelatinized starch and talcum powder are evenly mixed and pressed into tablets.
Embodiment 3
[0037] prescription:
[0038]
[0039] Preparation Process:
[0040] Weigh the prescription amount of dextran, silicon dioxide, hydrogenated castor oil and limatoprost alfa cyclodextrin inclusion compound, disperse with appropriate amount of tert-butanol-water system (volume ratio 1:4), freeze-dry, and pre-freeze The temperature is -45°C, the primary drying temperature is -20°C, and the secondary drying temperature is 15°C; the freeze-dried block is crushed and passed through a 80-mesh sieve to obtain a freeze-dried powder; then mixed with the prescribed amount of lactose, microcrystalline Cellulose, croscarmellose sodium and stearic acid are uniformly mixed and compressed into tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com