Composition of gonadotropic hormone releasing hormone analogue
A gonadotropin and hormone-releasing technology, which is applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems of expensive kits, affecting the accuracy of low-concentration quantitative results, and radioactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
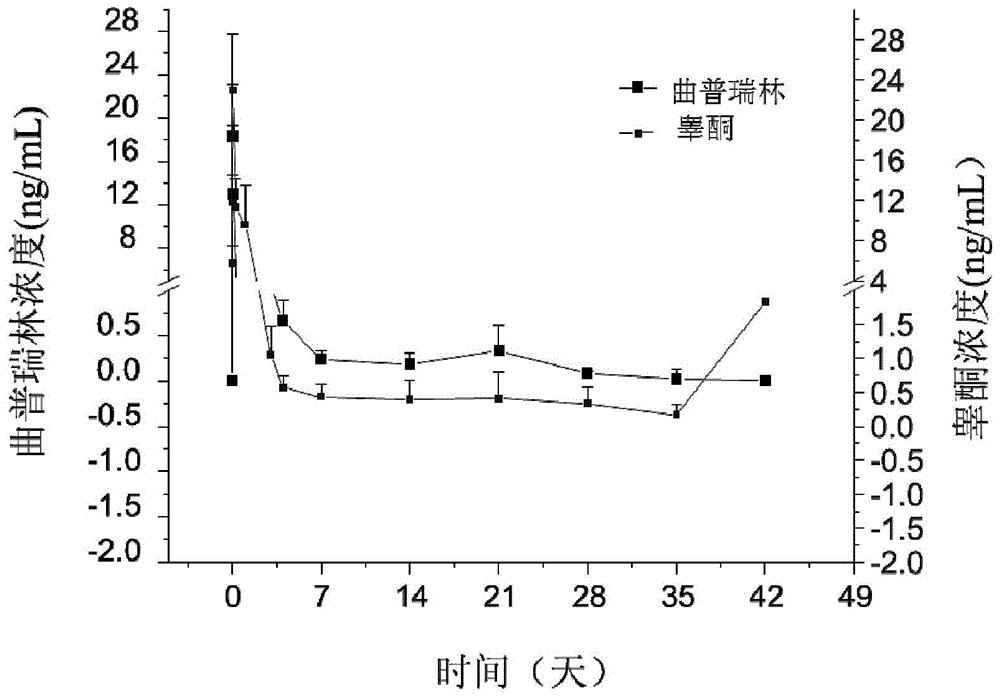
Examples
Embodiment 1
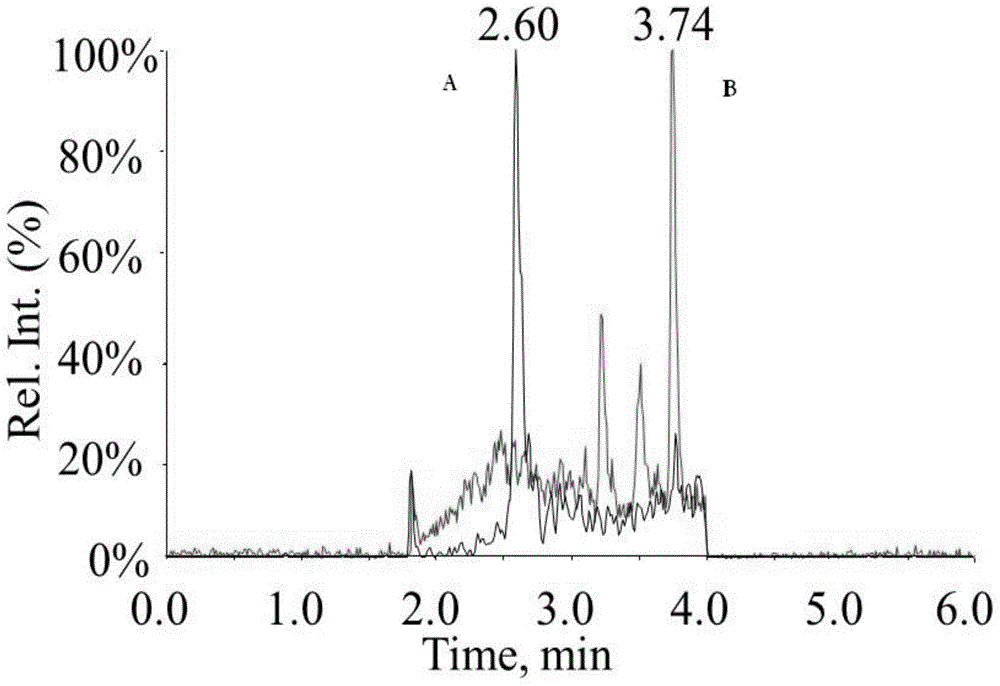
[0024] Example 1 mobile phase is 0.02% propionic acid aqueous solution-methanol detection goserelin and testosterone standard solution
[0025] Standard solution sample preparation
[0026]Precisely weigh the reference substance of goserelin acetate, and prepare a 1.00 mg / mL mother solution with methanol solution for later use. Testosterone stock solution (100 μg / mL) was purchased from Sigma, USA. Using methanol-water-formic acid (60:40:0.08v / v / v) as the diluent, dilute testosterone and goserelin into a standard mixture of the following concentrations, 0.02+0.005, 0.05+0.01, 0.1+0.03, 0.2+0.1 and 0.5+0.3ng / mL.
[0027] Liquid condition chromatographic column: ZORBAX Eclipse plus C 18 (2.1mm x50mm, 1.8μm, Stockport, UK) Mobile phase: A: 0.02% propionic acid aqueous solution, B: methanol; flow rate: 0.4mL / min, column temperature: 40°C, injection volume: 10μl. Agilent1290 high performance liquid chromatography system, including binary infusion pump, automatic sampler, column ...
Embodiment 2
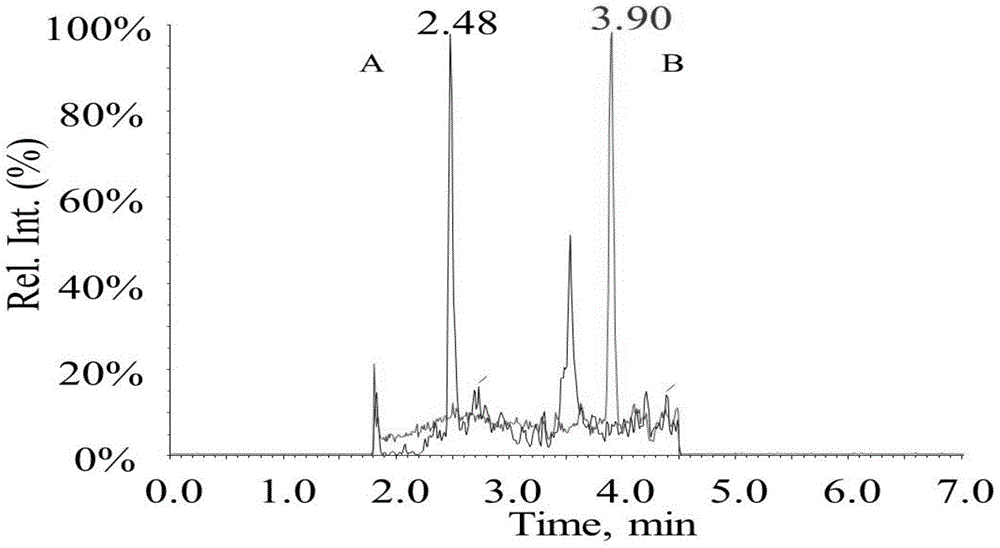
[0033] Example 2 The mobile phase is 0.02% propionic acid aqueous solution-methanol to detect goserelin and testosterone-d 3 Standard solution
[0034] The preparation of standard solution sample is the same as embodiment 1, and testosterone is replaced by testosterone-d during preparation 3 .
[0035] Liquid condition chromatographic column: ZORBAX Eclipse plus C 18 (2.1mm x50mm, 1.8μm, Stockport, UK); mobile phase: A: 0.02% propionic acid aqueous solution, B: methanol; flow rate: 0.4mL / min, column temperature: 40°C, injection volume: 10μl. Agilent1290 high performance liquid chromatography system, including binary infusion pump, automatic sampler, column thermostat. The gradient conditions are as follows:
[0036] Time(min)
A
B
0
90
10
1.8
90
10
2.2
35
65
2.8
30
70
3.8
30
70
4.5
8
92
5.0
90
10
7.0
90
10
[0037] Mass spectrometr...
Embodiment 3
[0040] Example 3 mobile phase is 0.05% propionic acid aqueous solution-methanol detection triptorelin and testosterone standard solution
[0041] The preparation of the standard solution sample was the same as in Example 1, except that goserelin was replaced by triptorelin.
[0042] Liquid conditions Chromatographic column: Eclipse plus RRHD C8 (2.1mm x50mm, 1.8μm, Agilent); mobile phase: A: 0.05% propionic acid aqueous solution, B: methanol; flow rate: 0.4mL / min, column temperature: 40°C, Sample volume: 10 μl. Agilent1290 high performance liquid chromatography system, including binary infusion pump, automatic sampler, column thermostat. The gradient conditions are as follows:
[0043] Time(min)
A
B
0
95
5
1.8
95
5
2.5
40
60
3.2
40
60
5.0
30
70
5.4
30
70
6.2
8
92
6.7
8
92
7.2
95
5
8.0
95
5
[0044] Mass...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Snr | aaaaa | aaaaa |
| Snr | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap