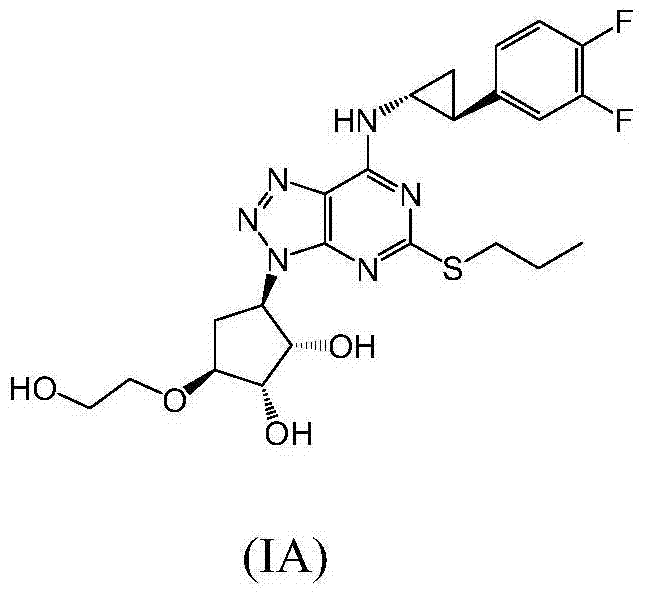
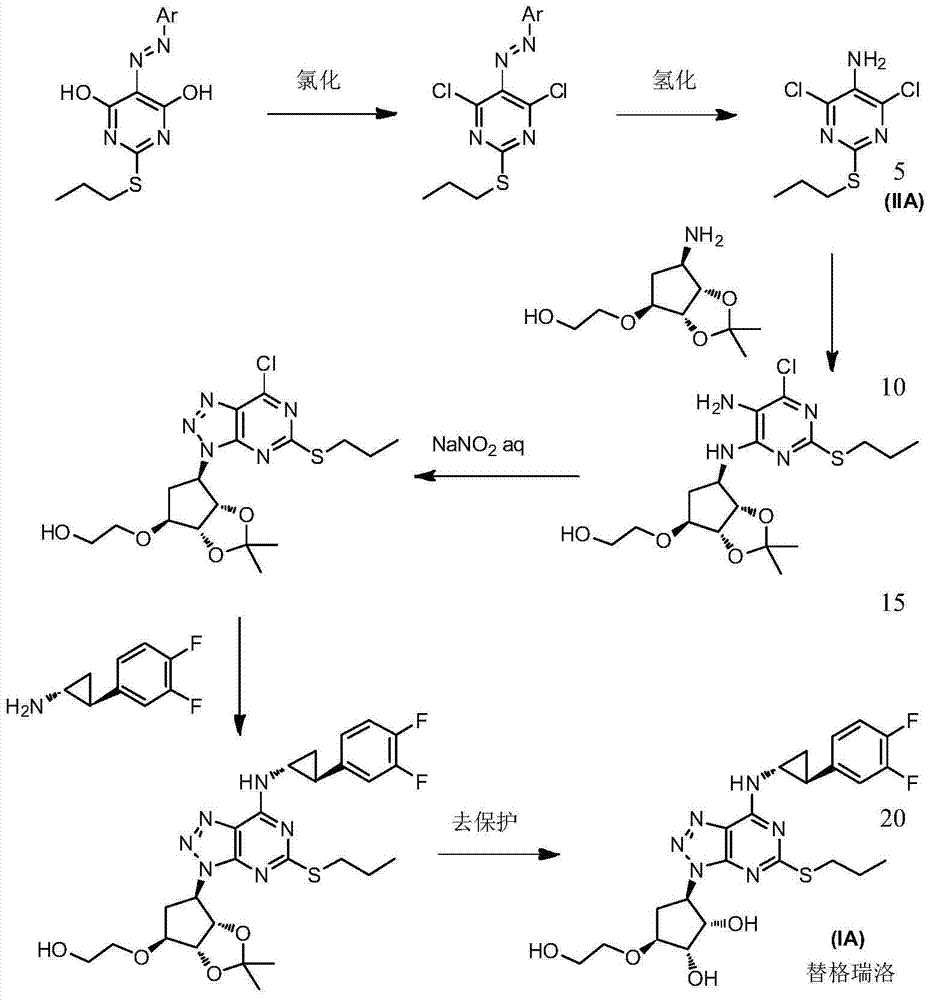
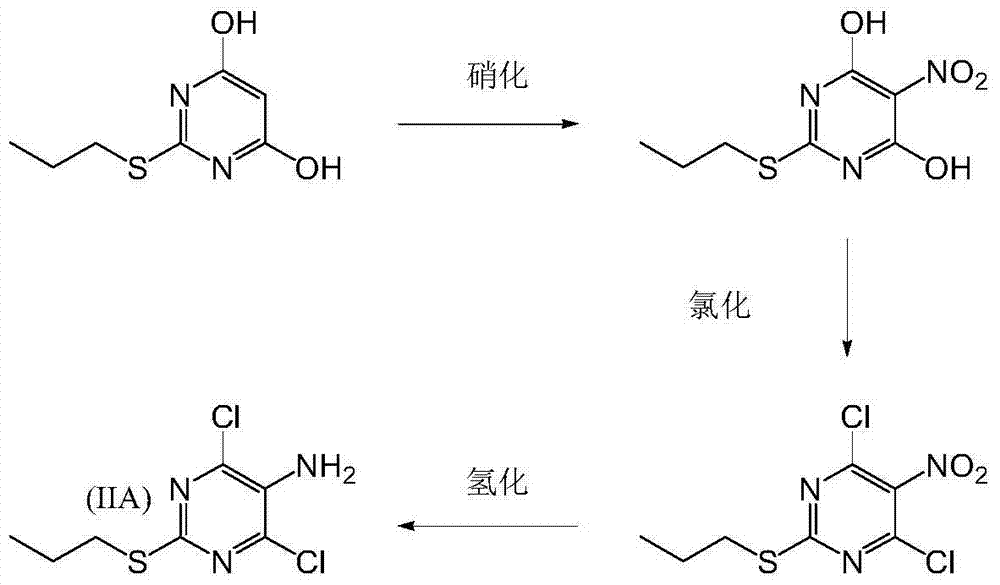
A process for the preparation of an intermediate for a triazolopyrimidine carbonucleoside
A compound and a selected technology, applied in the field of solvates and compounds of the formula for preparing ticagrelor, can solve problems such as difficulties in industrial scale expansion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0187] Example 1. Preparation of N-(4,6-dihydroxy-2-mercaptopyrimidin-5-yl)acetamide (compound of formula VA, wherein R 1 is methyl).
[0188]
[0189] Procedure for the separation of sodium salts.
[0190] Diethyl acetamidomalonate (5 g, 23.01 mmol) and thiourea (2.45 g, 32.2 mmol, 1.4 eq) were added to sodium ethoxide (21% w / w, 20 ml, 52.9 mmol, 2.3 eq) in EtOH (65 mL ) solution. The reaction mixture was heated to reflux temperature for 4h. The resulting suspension was cooled to RT, then to 0 °C, filtered, and the sodium solid salt was washed with EtOH (10 mL).
[0191] The sodium salt was dissolved in a minimum amount of water (25 mL), and the solution was acidified to pH 1 with concentrated HCl (4 mL). The resulting precipitate was filtered and washed with cold EtOH (5 mL) and cold EtOH 2 O (5 mL) wash. The solid was dried under vacuum to afford N-(4,6-dihydroxy-2-mercaptopyrimidin-5-yl)acetamide (3.24 g, 70% yield, 100% HPLC-MS) as a pale yellow solid.
[0192...
Embodiment 2
[0195] Example 2. Preparation of N-(4,6-dihydroxy-2-(propylthio)pyrimidin-5-yl)acetamide (compound of formula IVA, wherein R 1 is methyl).
[0196]
[0197] NaOH (50% w / w, 2.6mL, 50mmol, 5eq) aqueous solution was slowly added to N-(4,6-dihydroxy-2-mercaptopyrimidin-5-yl)acetamide (2g , 9.95 mmol) in MeOH (10 mL). The reaction mixture was stirred at RT for 30 min, and 1-bromopropane (2.6 mL, 30 mmol, 3 eq) was added dropwise, the resulting solution was stirred at RT overnight, and a solid precipitated. The solvent was evaporated and water (7 mL) was added to obtain a clear solution. Concentrated HCl (2.4 mL) was added and the resulting solid was filtered, washed with cold water (2 mL), and dried in vacuo to afford N-(4,6-dihydroxy-2-(propylthio)pyrimidin-5-yl) Acetamide (1.96 g, 82% yield, 97.8% HPLC-MS), as an off-white solid.
[0198] 1 H-NMR (DMSO-d6, 400MHz, ppm): 12.54(s, 1H, OH), 11.44(s, 1H, OH), 8.87(s, 1H, NH), 3.06(t, J=6.8Hz, 3H , CH 2 ), 1.94 (s, 3H, CH 3...
Embodiment 3
[0199] Example 3. Preparation of 5-amino-2-(propylthio)pyrimidine-4,6-diol hydrochloride (compound IIIA-HCl).
[0200]
[0201] 6M HCl (5 mL) was added to a suspension of N-(4,6-dihydroxy-2-(propylthio)pyrimidin-5-yl)acetamide (5 g, 20.55 mmol) in MeOH (20 mL) from Example 2 . The resulting suspension was stirred at 50 °C for 18 h and evaporated to dryness. Toluene was added to the residue, and the mixture was concentrated to dryness (3 x 20 mL) to give 5-amino-2-(propylthio)pyrimidine-4,6-diol (III-HCl) (5 g, quantitative yield Yield, 93.5% HPLC-MS), as a pale yellow solid.
[0202] 1 H-NMR (DMSO-d6, 400MHz, ppm): 3.10(t, J=6.8Hz, 3H, CH 2 ), 1.65 (sex, J=6.8, 7.6Hz, 2H, CH 2 ), 0.95(t, J=7.6Hz, 3H, CH 3 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com