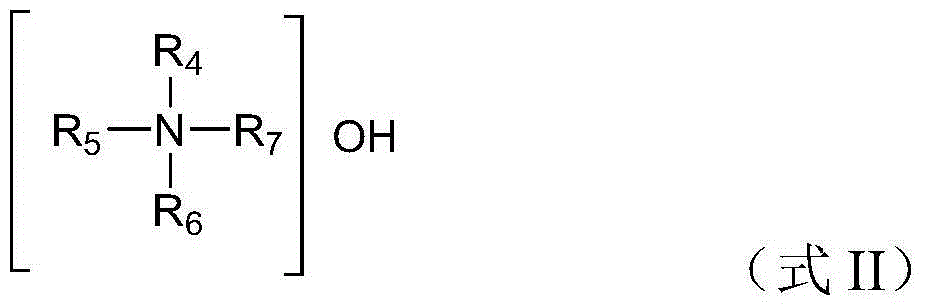Method for preparing dimethyl sulfoxide
A technology of dimethyl sulfoxide and dimethyl sulfide, which is applied in the field of preparation of dimethyl sulfoxide, can solve the problems of high cost, high price of oxidant, and large dosage, and achieve easy control, improved conversion rate, and selective The effect of sexual stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0012] The present invention provides a kind of preparation method of dimethyl sulfoxide, and this method comprises under oxidation reaction condition, in fixed bed reactor, make dimethyl sulfide and at least one kind of peroxide and at least one kind of The catalyst beds of the titanium-silicon molecular sieves are contacted to obtain a reaction mixture containing dimethyl sulfoxide, wherein at least part of the titanium-silicon molecular sieves are non-fresh titanium-silicon molecular sieves.
[0013] The titanium-silicon molecular sieve is a general term for a class of zeolites in which titanium atoms replace a part of the silicon atoms in the lattice framework, and can be represented by the chemical formula xTiO 2 ·SiO2 2 express. The present invention has no special limitation on the content of titanium atoms in the titanium-silicon molecular sieve, which can be a conventional choice in the field. Specifically, x may be 0.0001-0.05, preferably 0.01-0.03, more preferably...
Embodiment 1
[0066] The catalyst C1 used in this example is obtained by regenerating the shaped titanium-silicon molecular sieve TS-1 (average particle size: 55 μm) unloaded from the cyclohexanone ammoximation reaction device, wherein the regeneration conditions are: at 550 ℃ in the air atmosphere for 4h. Based on the total amount of shaped titanium-silicon molecular sieve TS-1, the content of titanium-silicon molecular sieve TS-1 is 85% by weight, and the content of silicon oxide is 15% by weight; the activity after regeneration is 50% (its activity when fresh is 95%).
[0067] Catalyst C1 was packed in a stainless steel fixed-bed microreactor (loading volume: 15 mL, reactor height-to-diameter ratio: 15) to form a catalyst bed, where the number of catalyst beds was one.
[0068] Dimethyl sulfide, hydrogen peroxide and methanol are combined to form a liquid mixture. Aqueous ammonia (concentration: 25% by weight) was added to the liquid mixture to adjust the pH of the liquid mixture to 6....
Embodiment 2
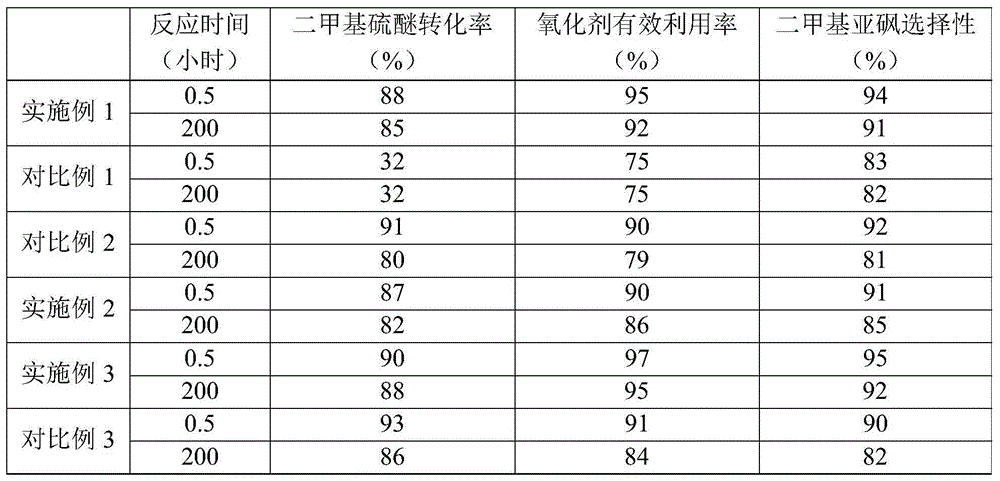
[0076] Dimethyl sulfoxide was prepared by the same method as in Example 1, except that ammonia water was not added to the liquid mixture (the pH of the liquid mixture was 5.8). The reaction mixture obtained at 0.5 hour and 200 hours after the start of the reaction was analyzed by gas chromatography, and the conversion rate of dimethyl sulfide, the effective utilization rate of the oxidant and the selectivity of dimethyl sulfoxide were calculated. The results are listed in Table 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Radial length | aaaaa | aaaaa |
| Benzene adsorption | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com