A kind of test kit for typing detection of Helicobacter pylori
A technology for Helicobacter pylori and bacterial protein, which is applied in measurement devices, analytical materials, instruments, etc., can solve problems such as HP typing and typing, and achieve the effects of small sample demand, high sensitivity and wide application range.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
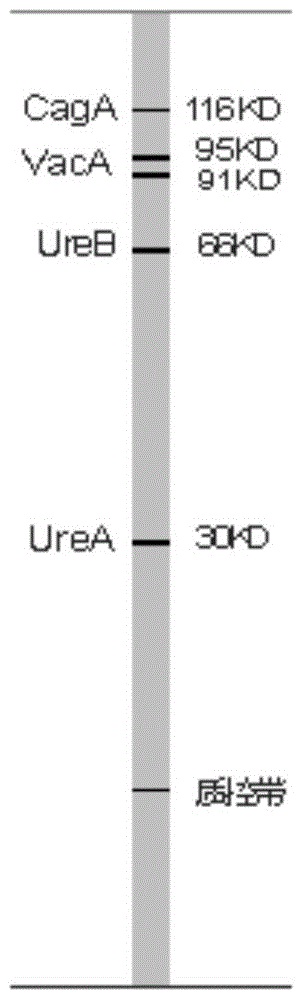
[0044] Example 1: Preparation of Helicobacter pylori (HP) blotting membrane
[0045] 1. HP whole cell protein electrophoresis separation
[0046] 1. Configure sample buffer: Use a sample gun to draw 10 mL of 0.05M pH7.4 Tris-HCL, 0.5 mL of 2-mercaptoethanol, 2 mL of glycerol, 0.5 mL of 0.1% bromophenol blue, and 2 mL of 10% sodium lauryl sulfate. Mix well and get it.
[0047] 2. Dilute HP whole cell protein with sample buffer to 0.04±0.005mg / mL, treat it at 100°C for 3 minutes, and cool it at 4°C for later use.
[0048] 3. Gradient discontinuous electrophoresis
[0049] 3.1. Preparation of separation gel: measure 40% acrylamide 4mL, 40% methylene-bisacrylamide 0.1mL, pH9.2 0.5M Tris-HCL 5mL, DW 5mL, TEMED (tetramethylethylenediamine) 50μL, 1 % Ammonium persulfate 5mL, H 2 O 12.5mL.
[0050] 3.2 Glue filling: Measure 30±0.5mL with a graduated cylinder, pipette 1mL of n-butanol, add it to the glue and seal it for solidification.
[0051] 3.3 After the separation gel has solidified (20±2 m...
Embodiment 2
[0061] Example 2: The kit of the present invention
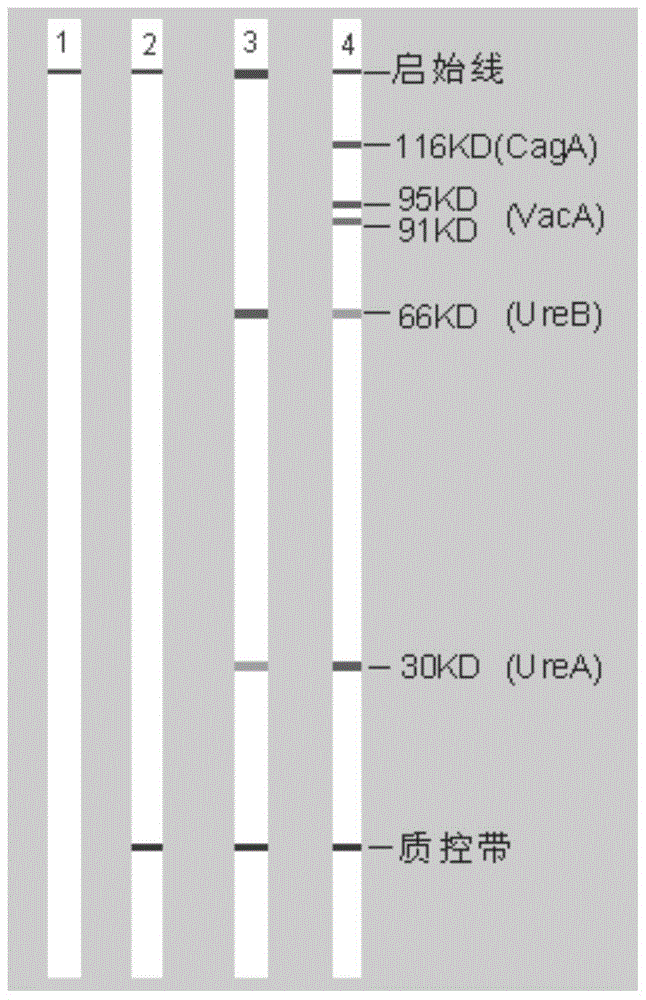
[0062] A kit for typing and detecting Helicobacter pylori includes the imprinted membrane prepared in Example 1, each with a length of 98±2mm and a width of 150±5mm.
Embodiment 3
[0063] Example 3: The kit of the present invention
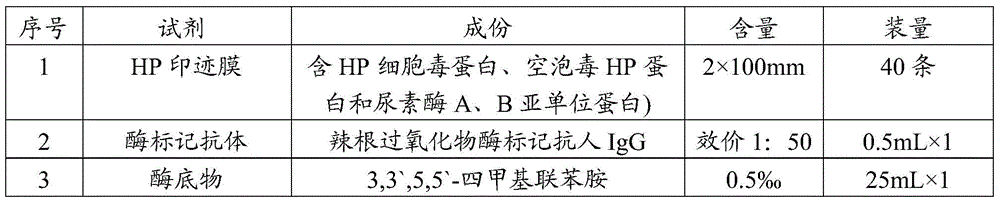
[0064] A kit for typing detection of Helicobacter pylori includes the blotting membrane prepared in Example 1, an enzyme-labeled antibody and an enzyme substrate, as shown in Table 1.
[0065] Table 1 Kits for typing detection of Helicobacter pylori
[0066]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com