B cell immunodominance epitope peptide of staphylococcus aureus enterotoxin B and preparation method and application thereof
A technology of staphylococcus intestinal and immune superiority, applied in the application field of medical biotechnology, can solve the problems of long test period, incomplete antigen B cell epitopes, complicated steps, etc., and achieves no omission of epitopes, good application prospects, and methods Easy and fast effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Example 1 Synthesis of Short Peptides Containing 18 Amino Acids with Walking Overlap
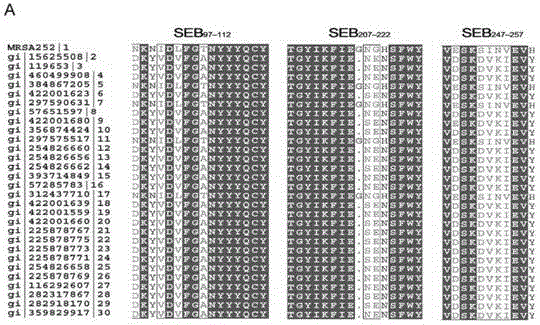
[0065] SEB is Staphylococcus aureus enterotoxin B, and its sequence is conserved among various strains, with a total length of 261 amino acids, of which 1-27 amino acids are signal peptides. The overlapping peptides constructed by recombination are 18 peptides between 1-261, which are derived from the MRSA252 international standard strain. The SEB protein sequence (number Q6GFN0) derived from Staphylococcus aureus MRSA252 was searched in the UniProt protein database, and a short peptide of 18 amino acids with overlapping steps was synthesized from the first amino acid (synthesized with the assistance of Shanghai Qiangyao Company), a total of 42 , with a purity greater than 90%. The synthetic peptide information is shown in Table 1. The synthesized peptides were dissolved in DMSO to a storage concentration of 0.5 mg / mL, and stored at -70°C after aliquoting.
[0066] UniProt protei...
Embodiment 2
[0072] Example 2 Screening and Identification of Antigen Dominant Epitope Peptides
[0073] 1. With the assistance of aluminum salt adjuvant, the protective effect analysis of antigen immunization and the collection of antiserum
[0074] (1) Preparation and identification of recombinant mutant SEB:
[0075] According to literature reports, three amino acid positions (L45R, Y89A, Y94A) in SEB were mutated, pGEX6p-2 vector was selected, and recombinant SEB protein (rSEB) was cloned and expressed. After the rSEB protein was purified by GST column (protein purity greater than 90%), It was identified by Western Blot. (Mutation site reference: Oral Vaccine Formulations Stimulate Mucosal and Systemic Antibody Responses against Staphylococcal Enterotoxin B in a Piglet Model. Clinical and Vaccine Immunology, 2010(17) 8: 1163-1169)
[0076] (2) Animal immunity:
[0077]Animals in aluminum salt adjuvant group were immunized by intramuscular injection, and animals in Freund's adjuvan...
Embodiment 3
[0091] Example 3 Synthesis of truncated peptides corresponding to step-overlapping immunodominant peptides
[0092] For the immunodominant epitope peptide sequence screened in Example 2, 2 amino acids were truncated sequentially from the N-terminal and C-terminal respectively, and the corresponding truncated peptides covering the full length of each immunodominant epitope peptide were synthesized; the recombinantly constructed overlapping peptides There are three groups of truncated peptides, each group corresponds to an immunodominant epitope peptide, and the difference between adjacent truncated peptides in each group is 2 amino acids, and the synthetic purity is greater than 90% (synthesized with the assistance of Shanghai Qiangyao Company). The synthetic truncated peptide information is shown in Table 3. The synthesized peptides were dissolved in DMSO to a storage concentration of 0.5 mg / mL, and stored at -70°C after aliquoting.
[0093] The amino acid sequence informat...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap