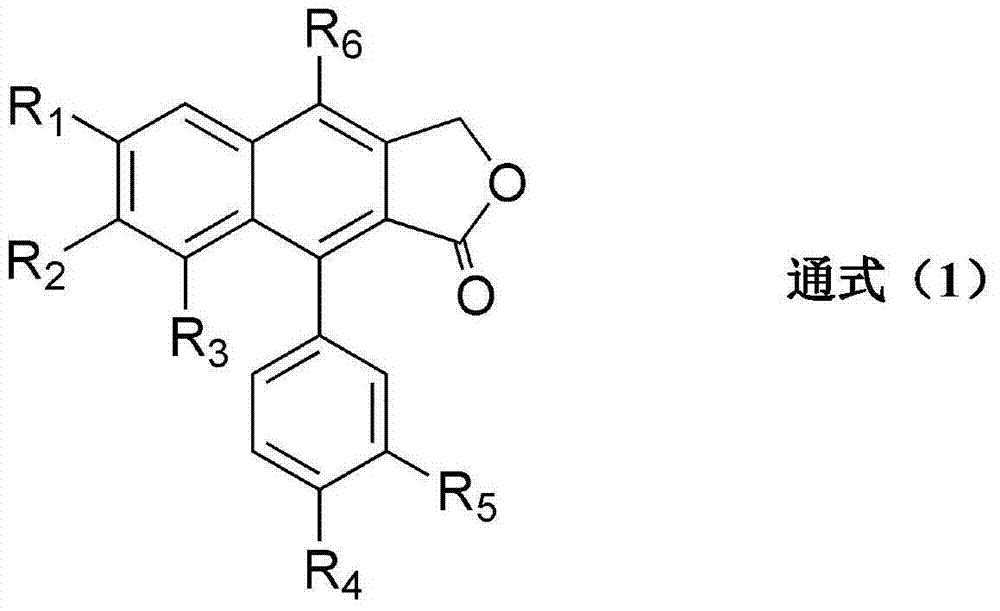
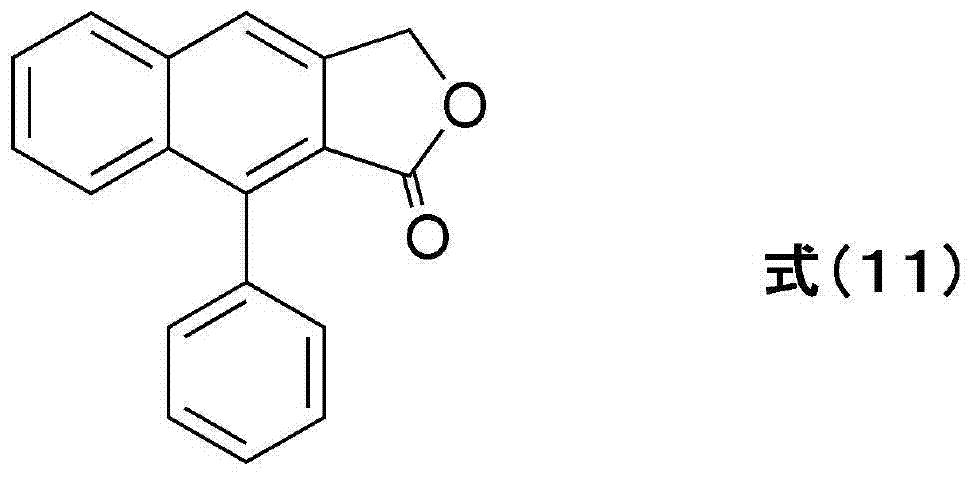
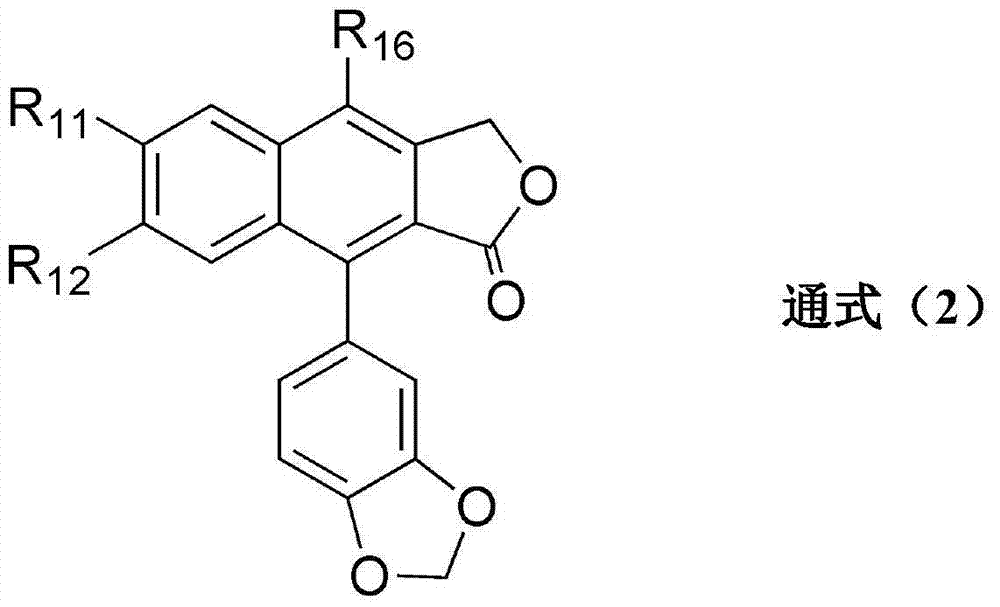
Transglutaminase activator
一种转谷氨酰胺酶、活化剂的技术,应用在转移酶、酶、化妆品等方向,达到促进表达、预防或改善皮肤粗糙、维持或改善屏障功能的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0098] In the preparation of the extract of acanthus or hydrangea, it is possible to use the acanthus or hydra as it is or to dry and pulverize the acanthus or hydra. In addition, steam distillates or pressed products of acanthus or hydrangea can also be used, refined products obtained by refining these with essential oils, etc. can also be used, and commercially available products can also be used. Acanthus or Hydra, or its steam distillate or pressed product can be used alone, or in combination of two or more.
[0099] The extraction solvent used in the preparation of the extract of Acanthus or Hydrangea can be appropriately selected, and solvents commonly used in the extraction of plant components can be listed, such as water; alcohols such as methanol, ethanol, propanol, butanol; Polyols such as ethylene glycol, propylene glycol, 1,2-butanediol, 1,3-butanediol, 1,4-butanediol, and 2,3-butanediol; ketones such as acetone and methyl ethyl ketone ; Esters such as methyl acet...
preparation example 1-1
[0360] 800 mL of 50 volume % ethanol aqueous solution was added to 80 g of the whole plant of Juechi (manufactured by Shinwa Bussan Co., Ltd.), and extraction was carried out at room temperature for 7 days. Thereafter, after filtering to obtain a crude extract, it was concentrated to dryness to obtain 6.6 g of extracted solids. This extracted solid content was dissolved in a 50 volume% ethanol aqueous solution so that the evaporation residual content was 1.0% (w / v), and a 50 volume% ethanol extract of acanthus was prepared.
preparation example 1-2
[0362] 500 mL of a 95% by volume ethanol aqueous solution was added to 50 g of the whole plant of Juechi (manufactured by Shinwa Bussan Co., Ltd.), and extraction was carried out at room temperature for 7 days. Thereafter, after filtering to obtain a crude extract, it was concentrated to dryness to obtain 897 mg of extracted solids. This extracted solid content was dissolved in 95 vol% ethanol aqueous solution so that the evaporation residual content was 1.0% (w / v), and a 95 vol% ethanol extract of Acanthus was prepared.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com